文献精选






This article is excerpted from the 《Frontiers in Sustainability》by Wound World




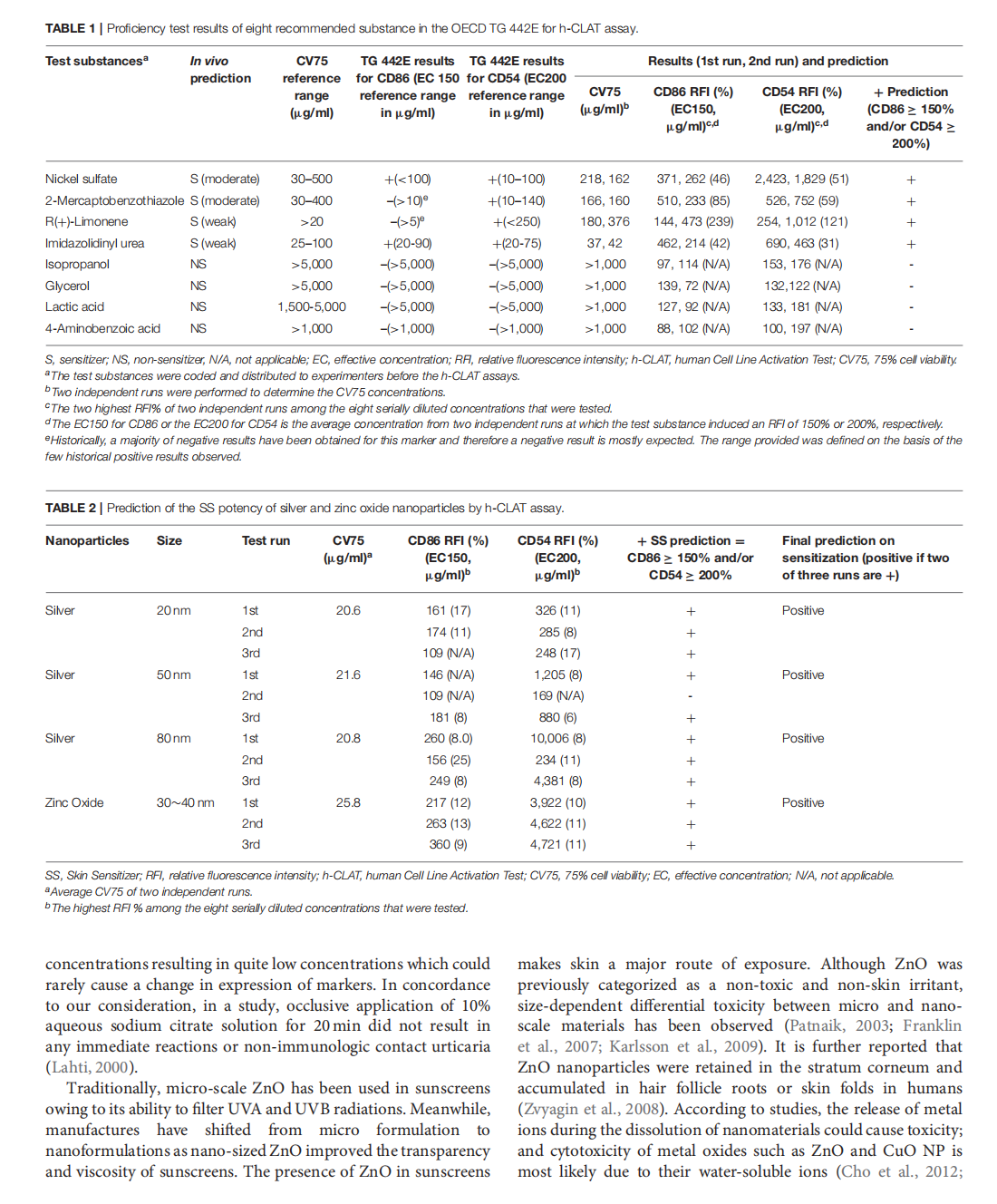
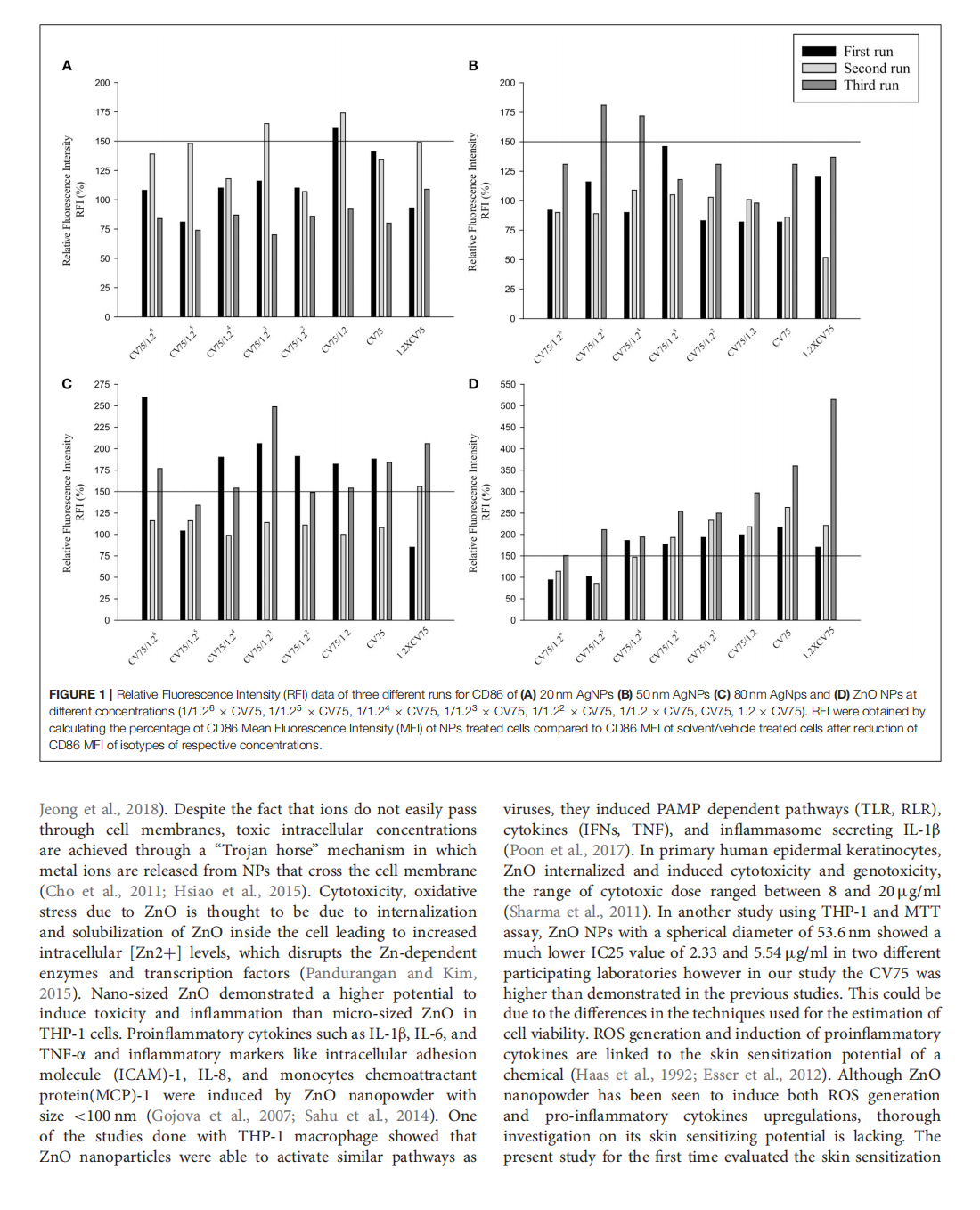
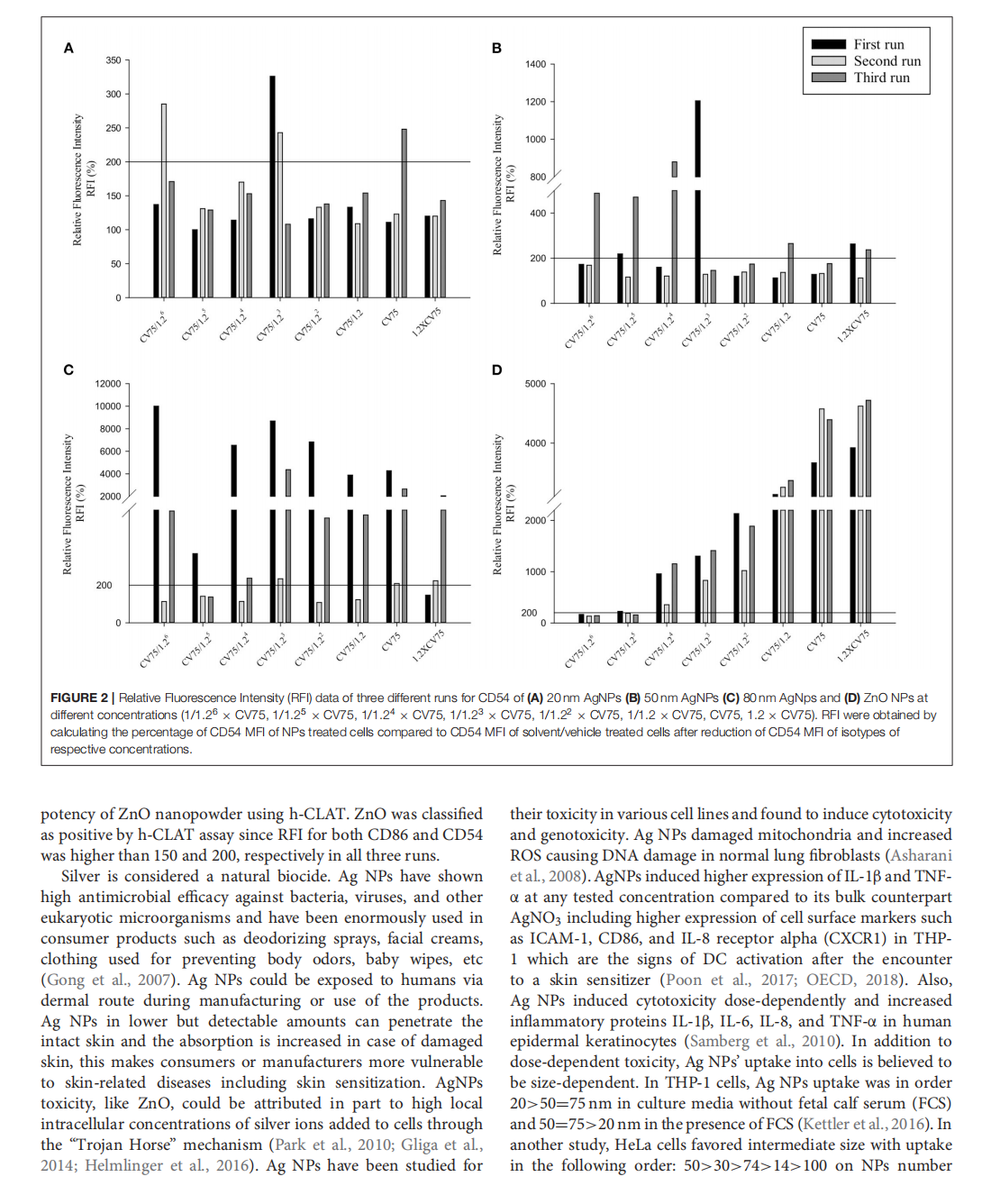



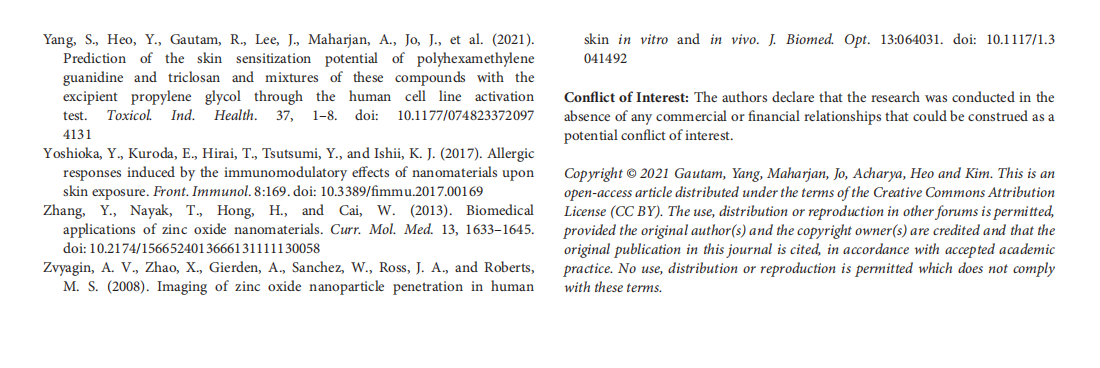
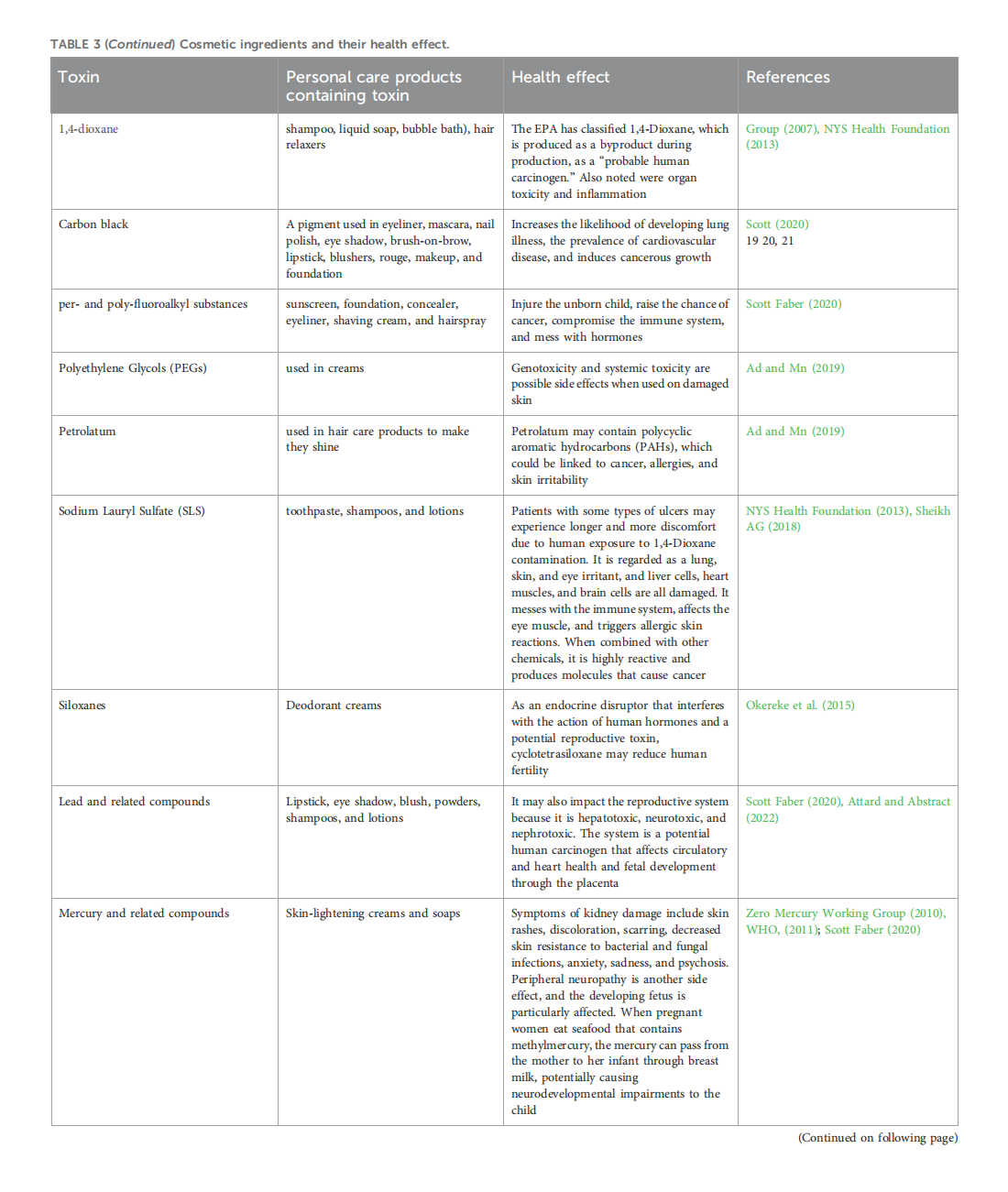
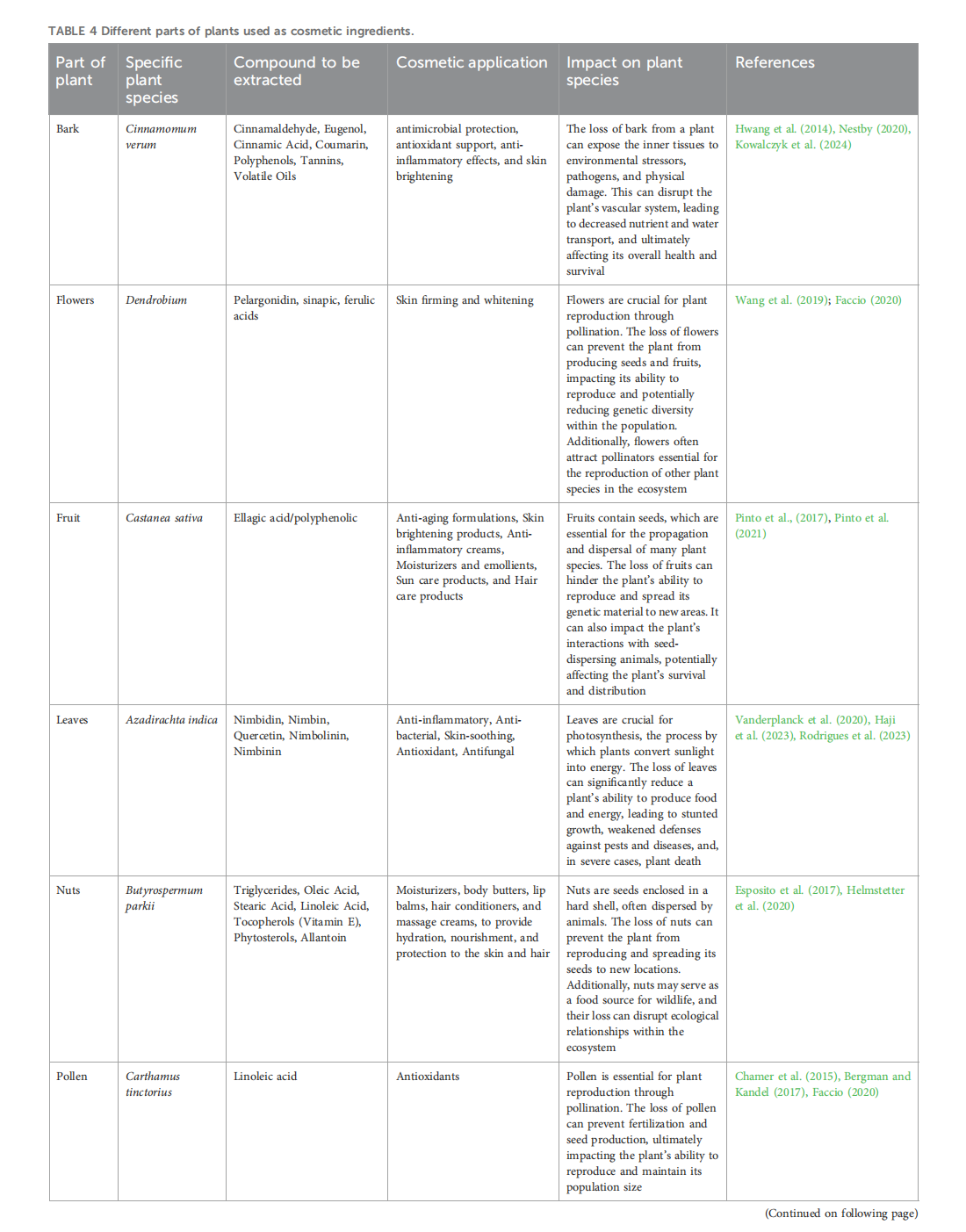
This article is excerpted from the 《Frontiers in Toxicology》 by Wound World




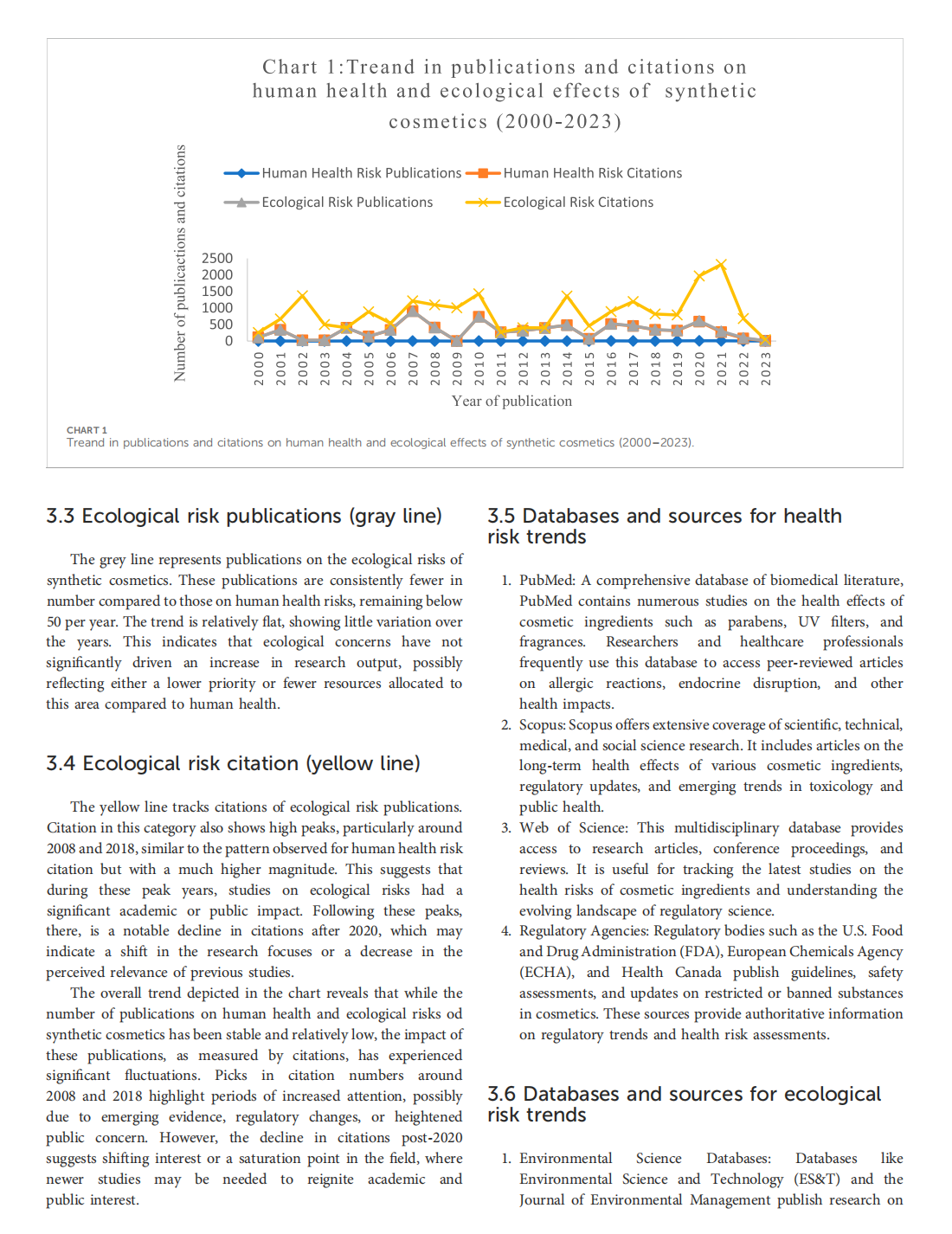
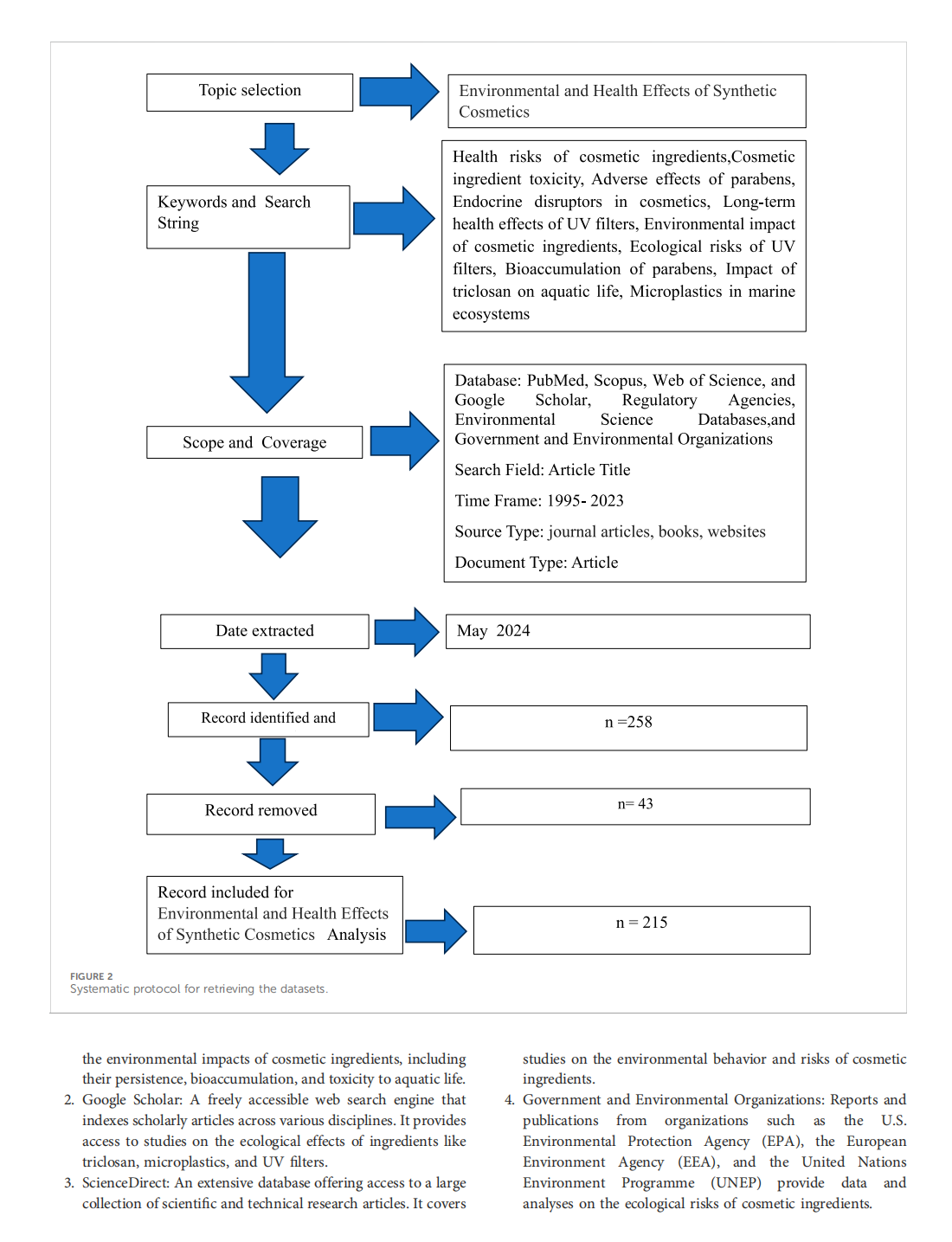





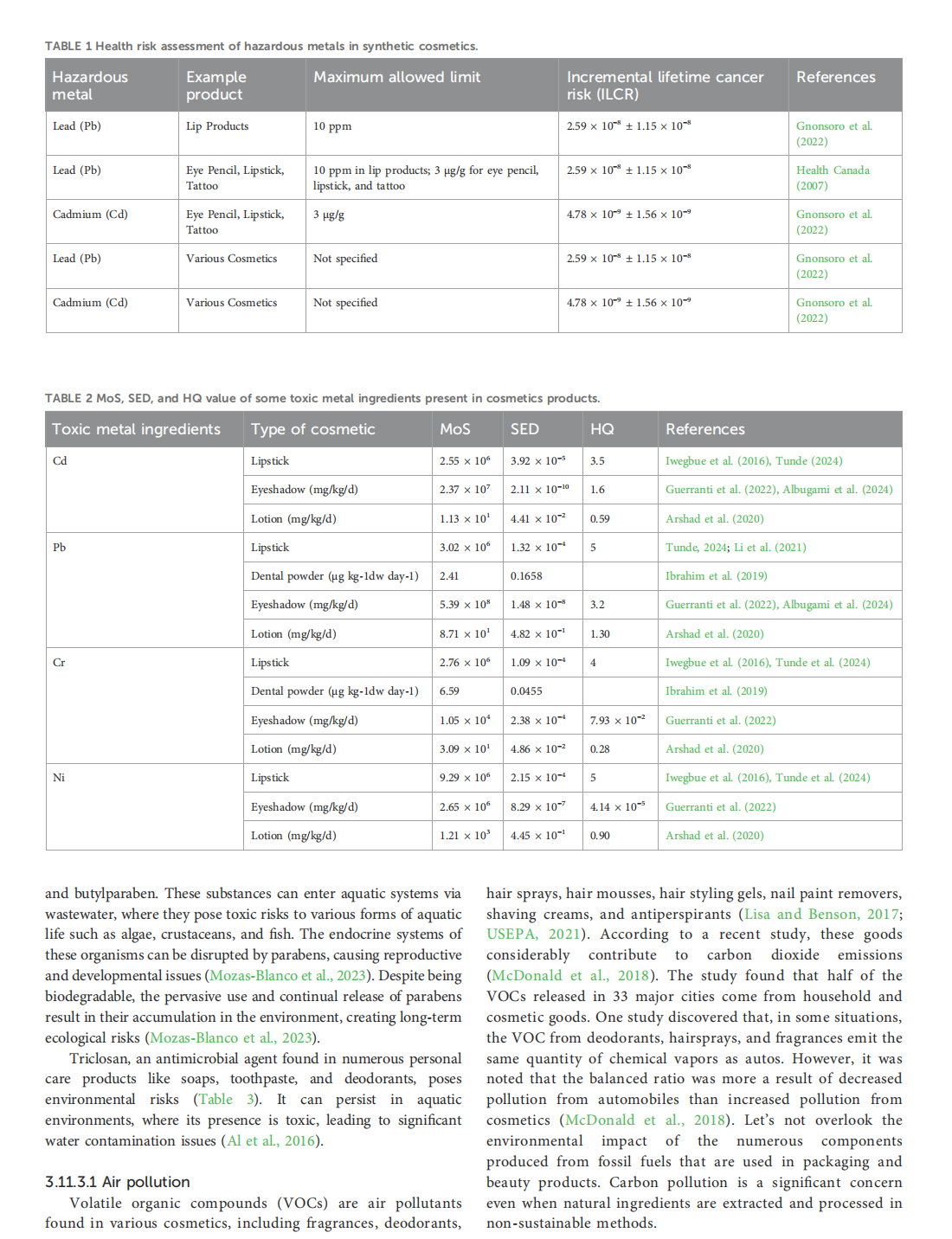
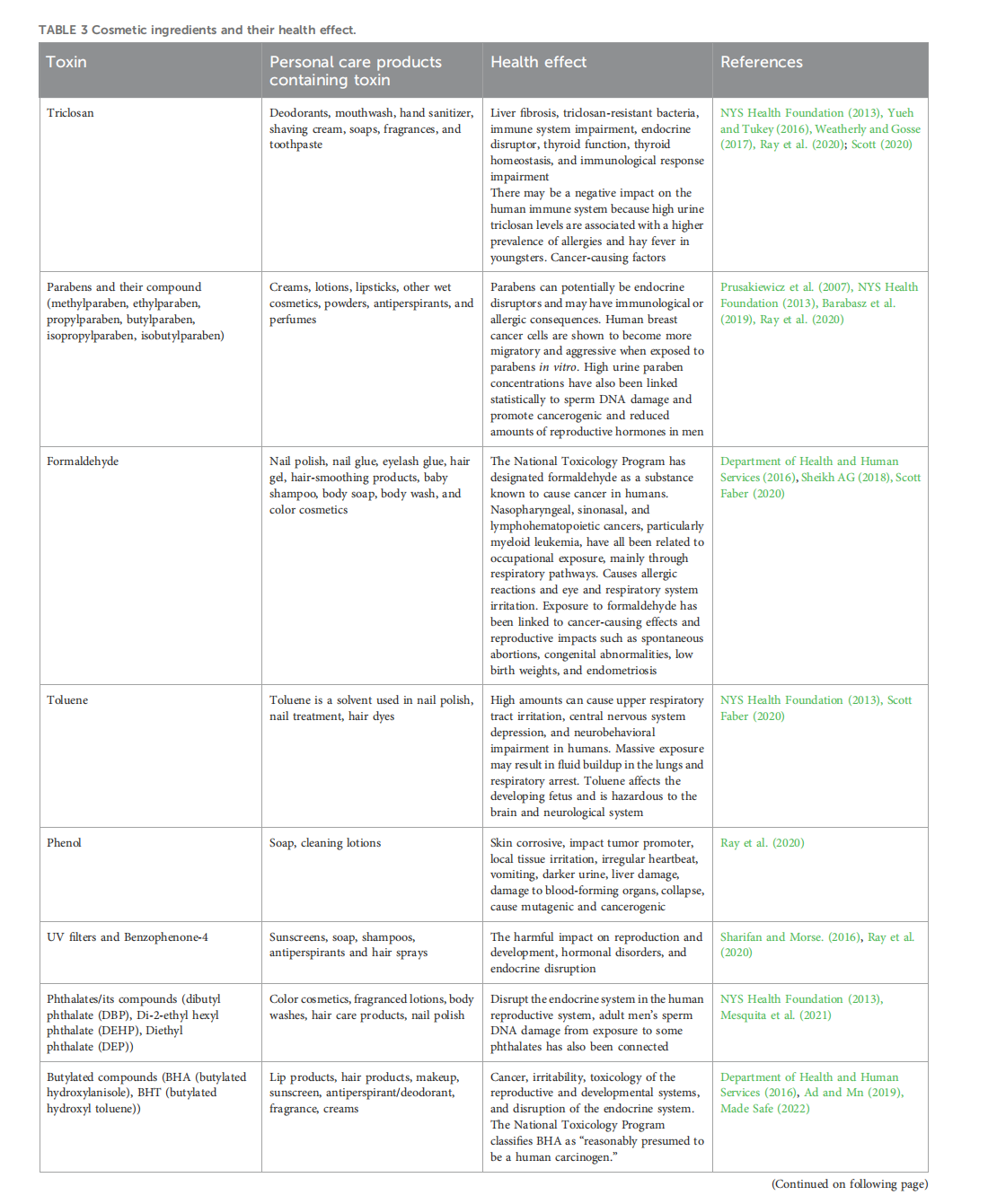





This article is excerpted from the《Frontiers in Environmental Science》 by Wound World 
The Role of Regional Contrast Changes and Asymmetry in Facial Attractiveness Related to Cosmetic Use





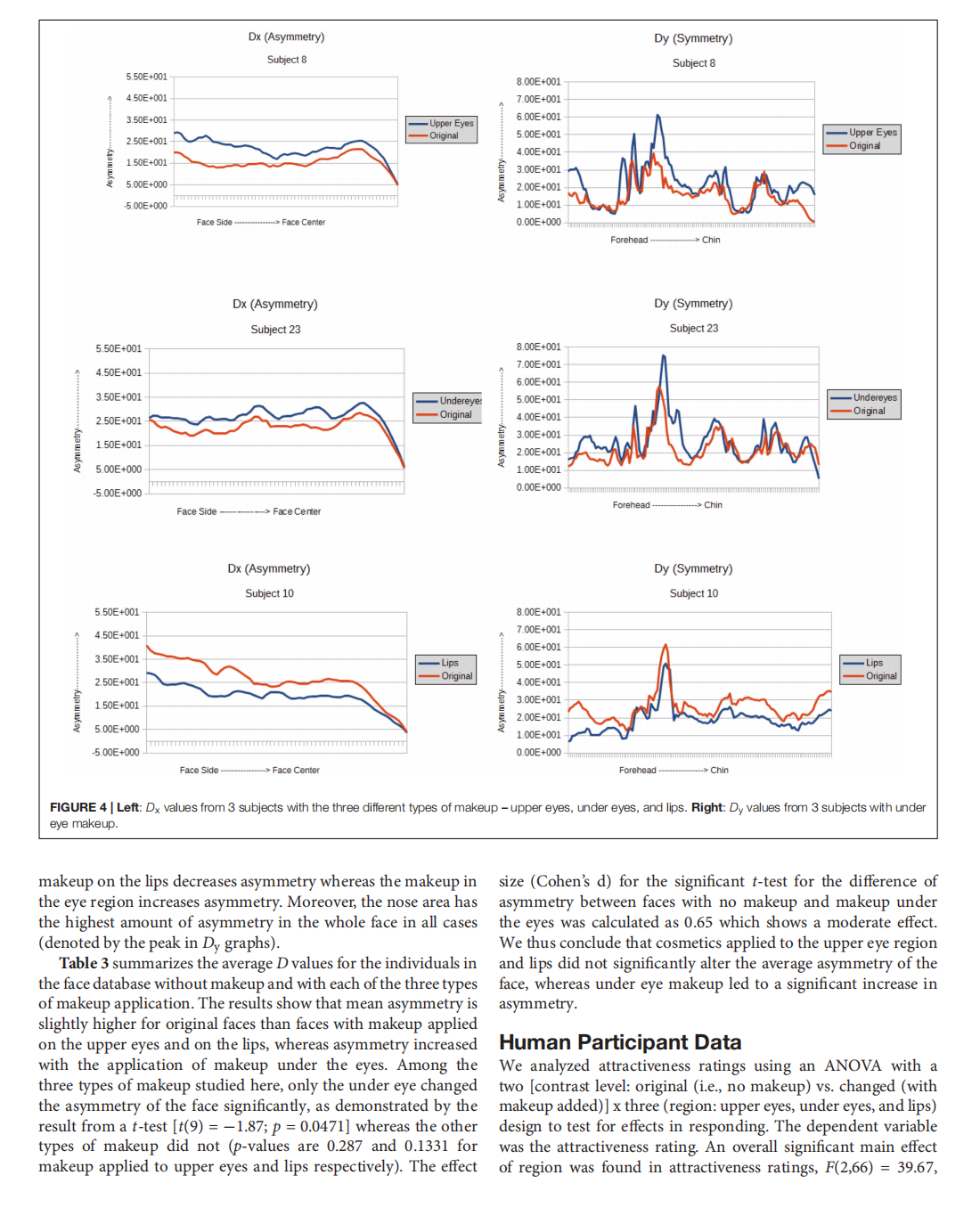
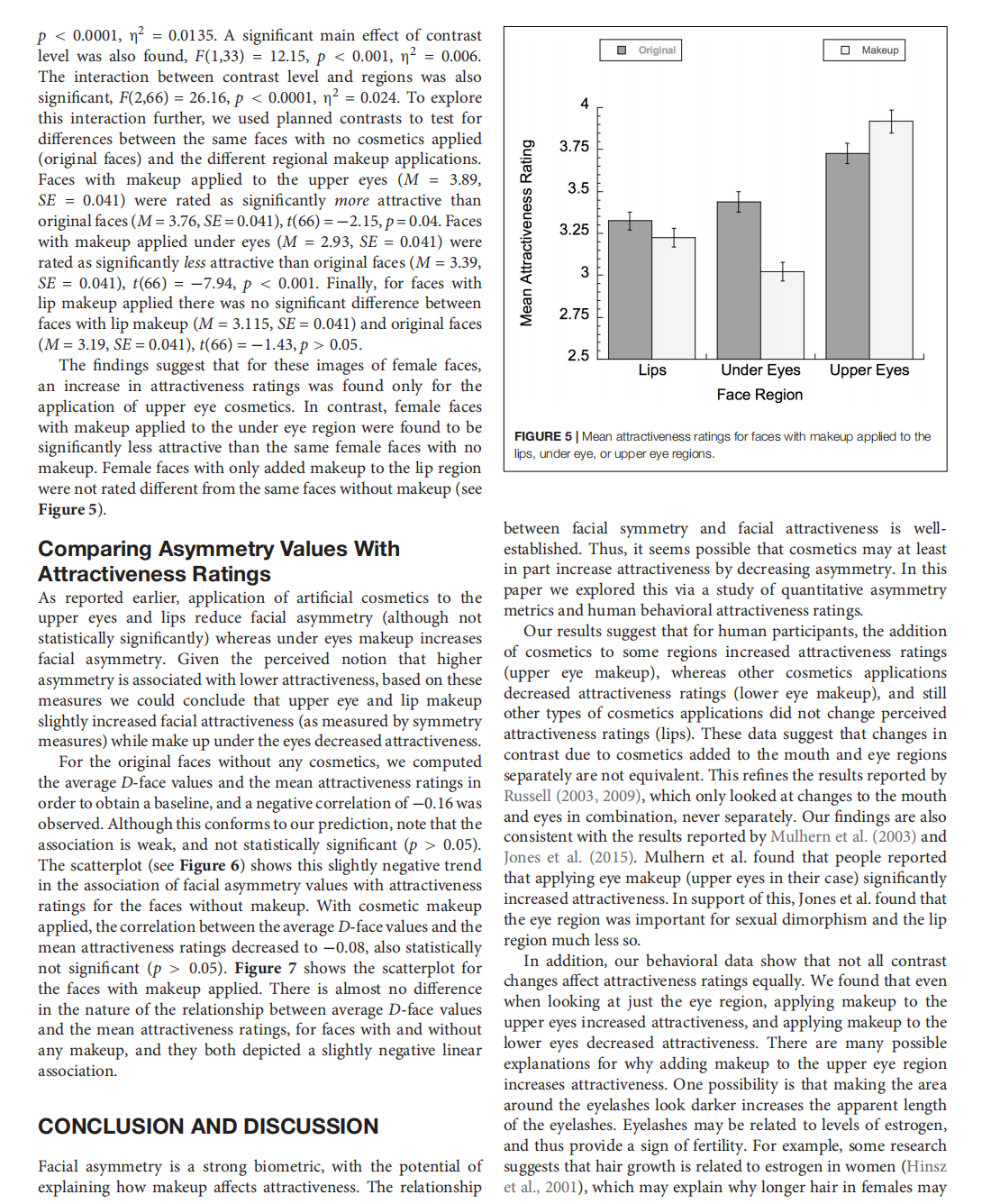



This article is excerpted from the 《Frontiers in Psychology》 by Wound World




