
伤口世界
电子邮件地址: 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。


- 星期六, 14 2月 2026
Deep learning techniques for imaging diagnosis of renal cell carcinoma: current and emerging trends

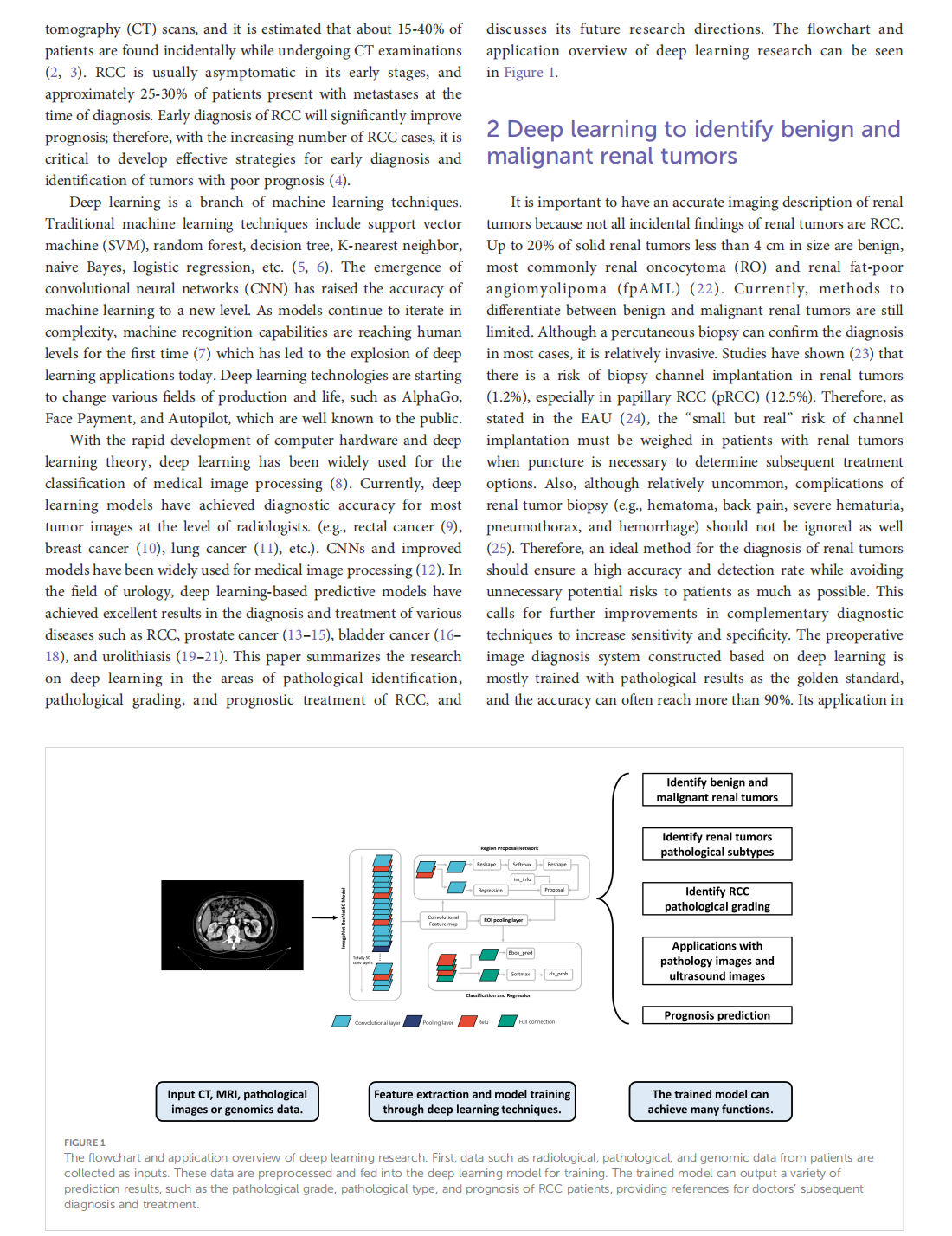
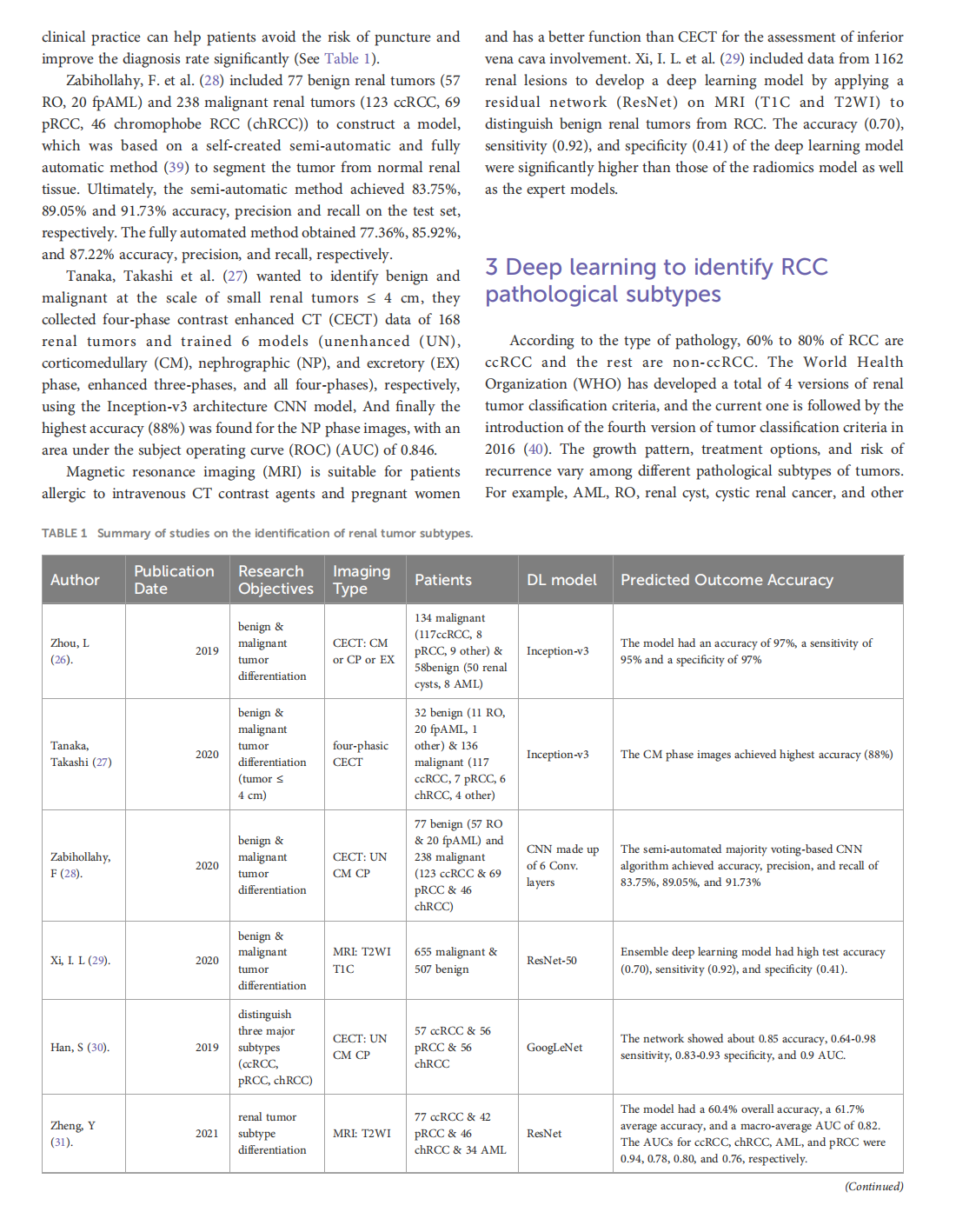
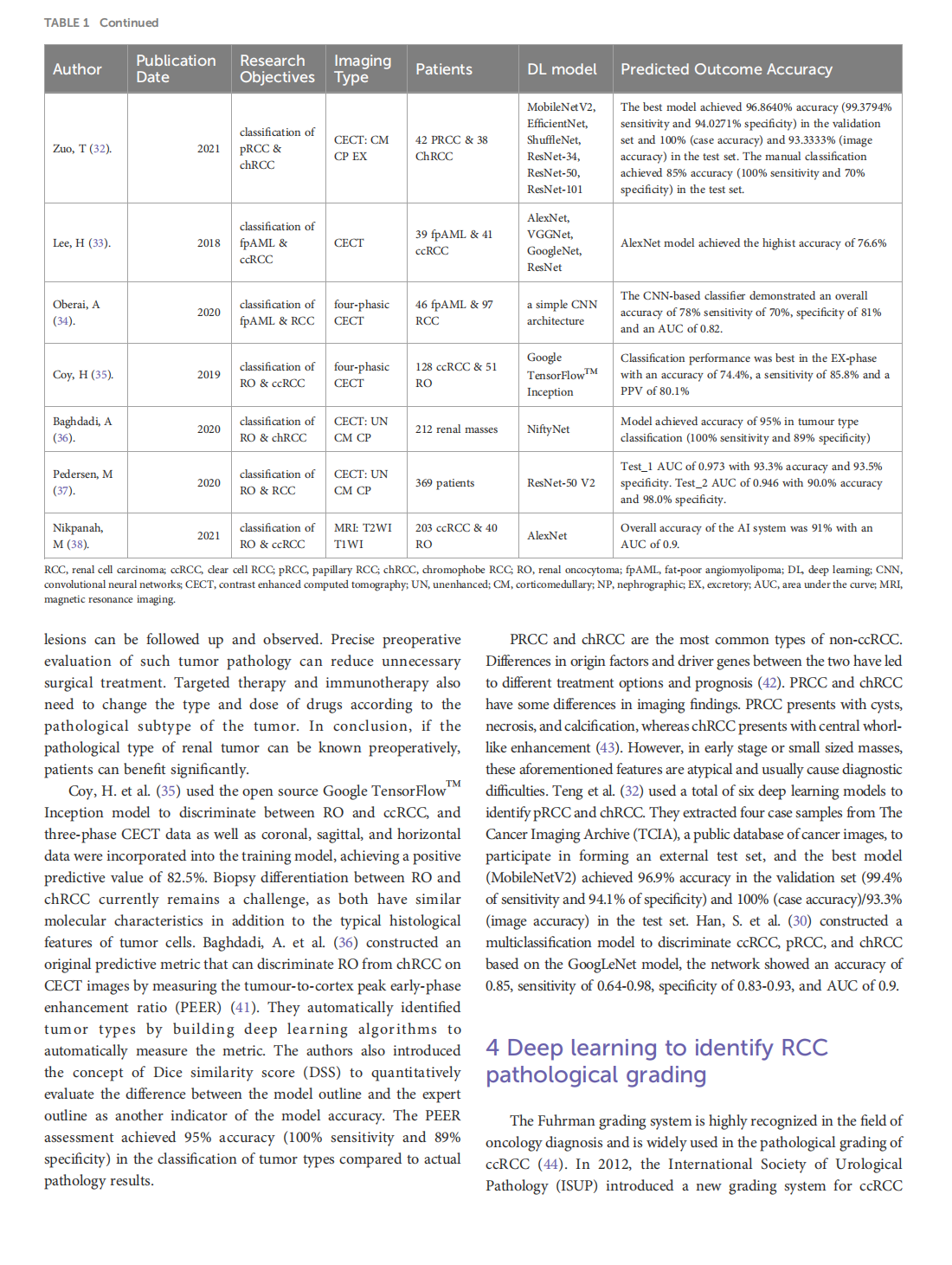
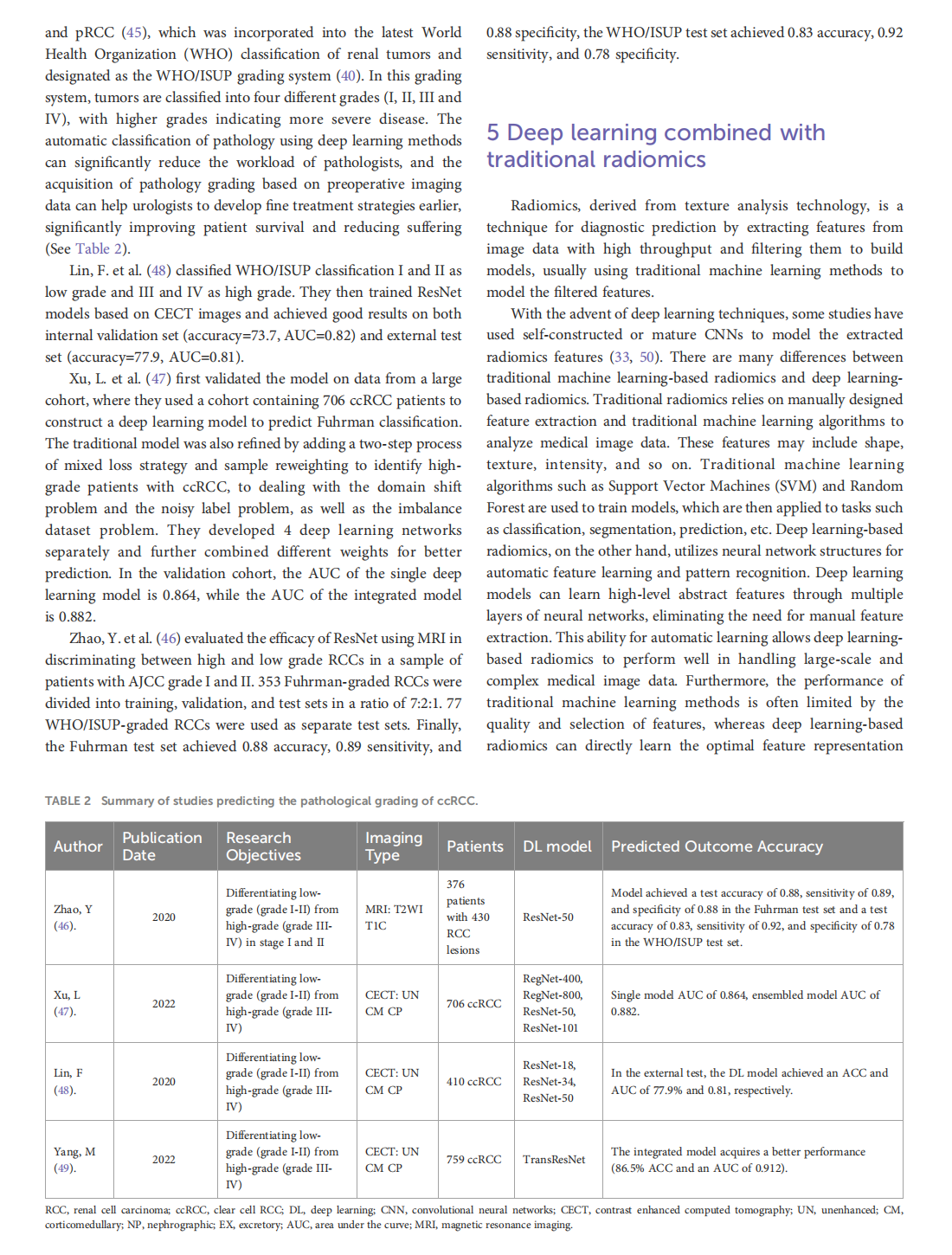
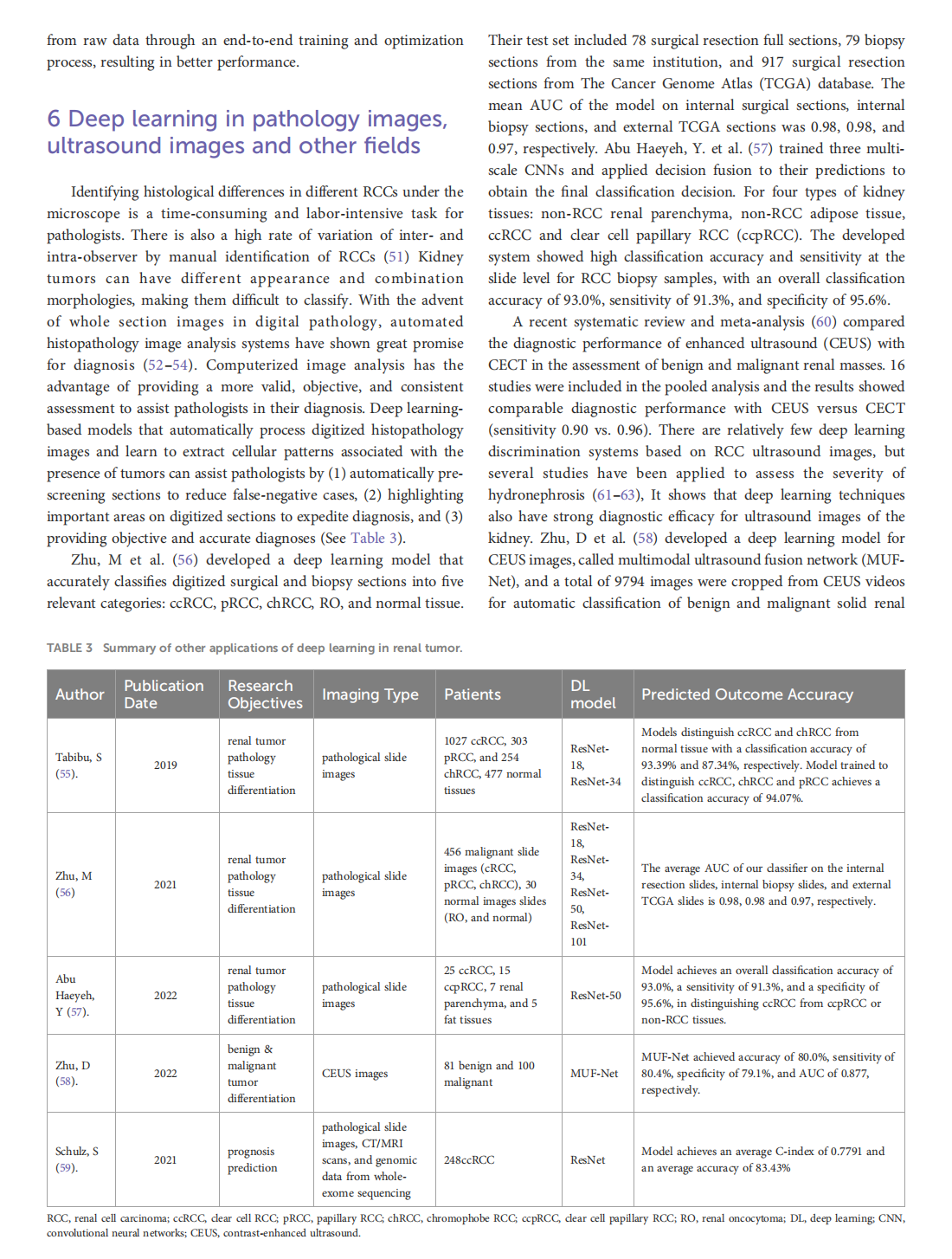





This article is excerpted from the《Frontiers in Oncology》by Wound World

- 星期三, 11 2月 2026
Medical treatment of acquired uterine arteriovenous fistulae related to pregnancy: 2 case reports and literature review

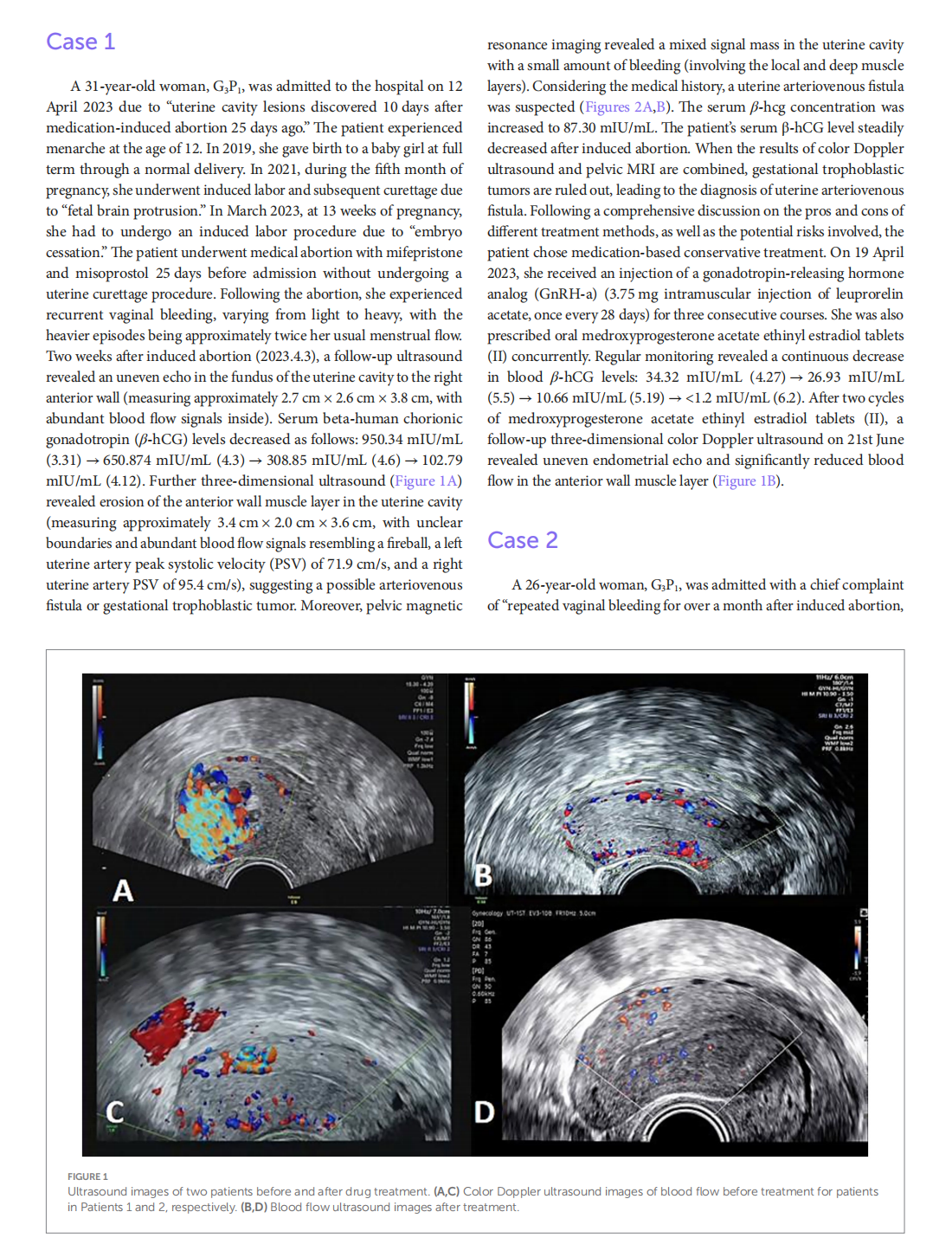

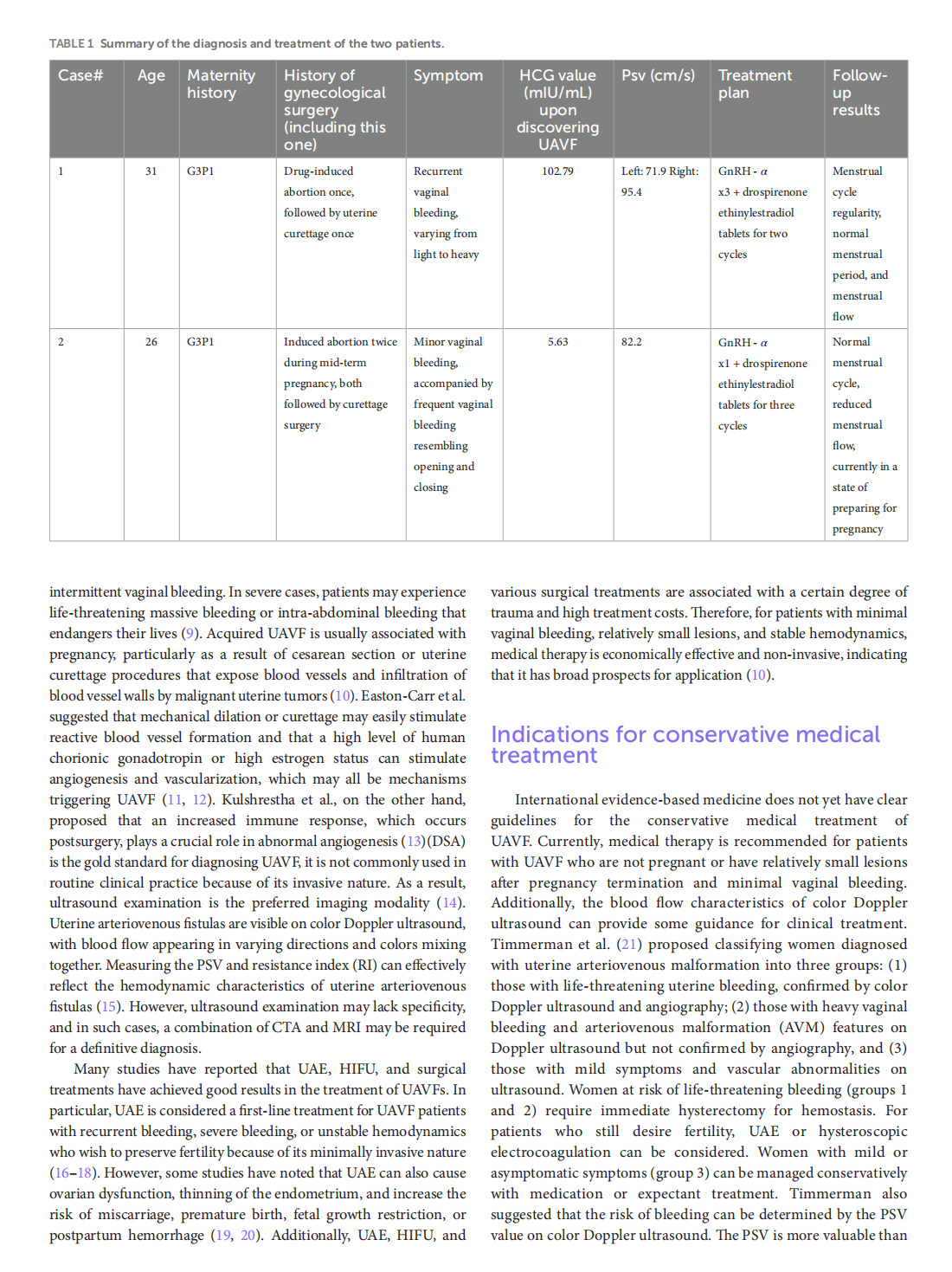



This article is excerpted from the 《Frontiers in Medicine》 by Wound World

- 星期一, 09 2月 2026
Virtual reality as a non-medical tool in the treatment of anxiety, pain, and perception of time in children in the maintenance phase of acute lymphoblastic leukemia treatment



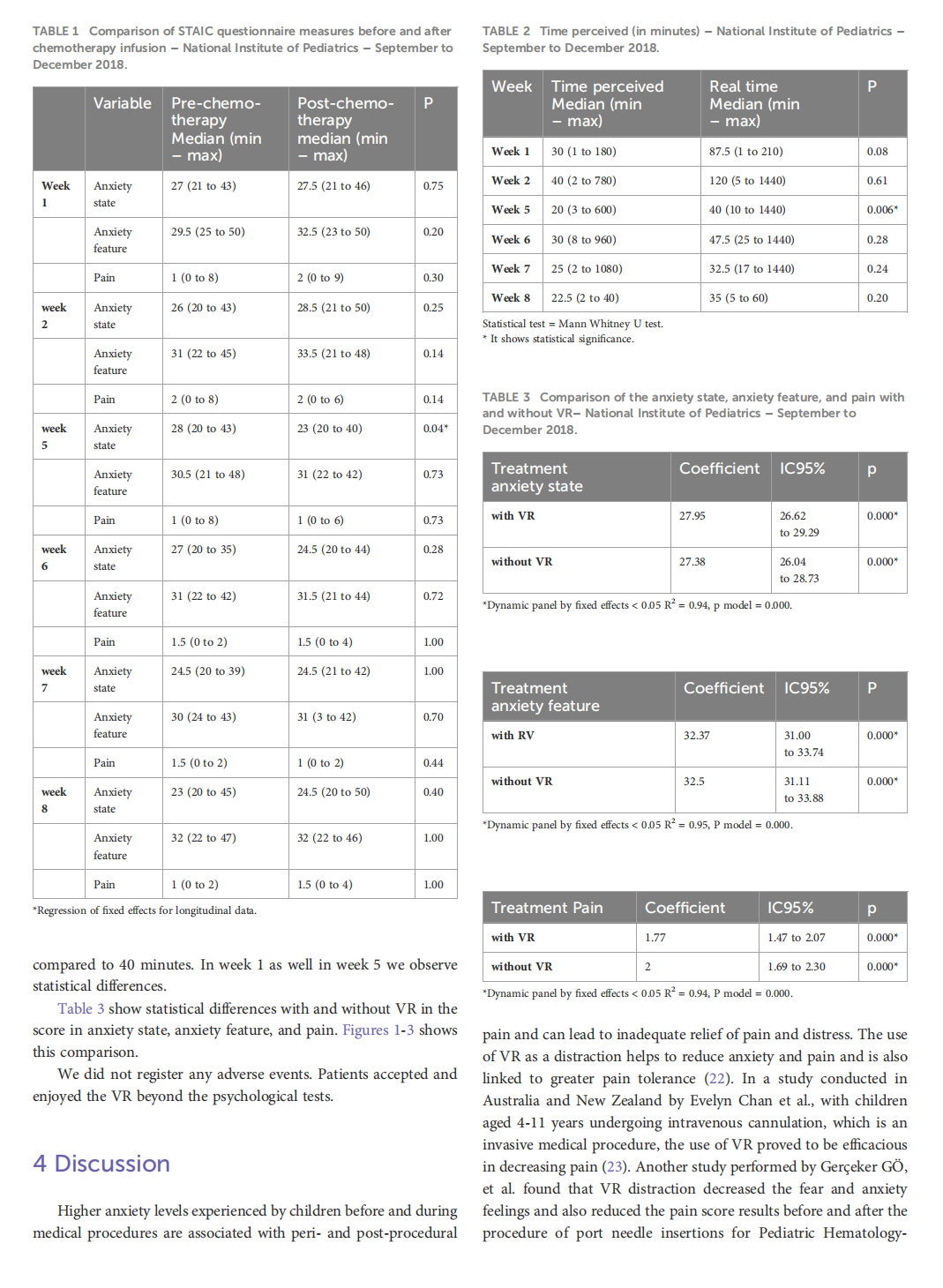
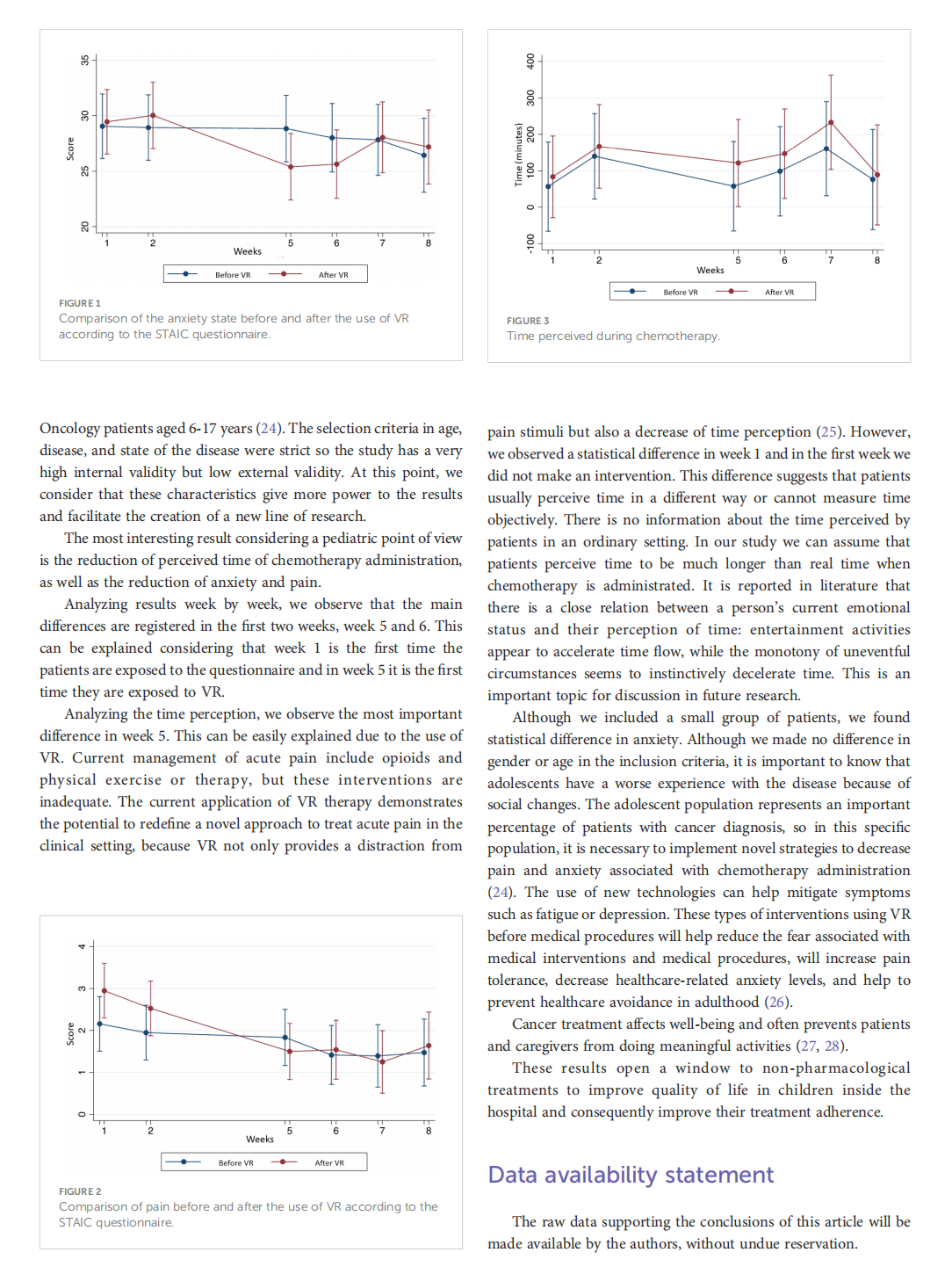


This article is excerpted from the《Frontiers in Oncology》by Wound World

- 星期五, 06 2月 2026
Virtual reality as a non-medical tool in the treatment of anxiety, pain, and perception of time in children in the maintenance phase of acute lymphoblastic leukemia treatment



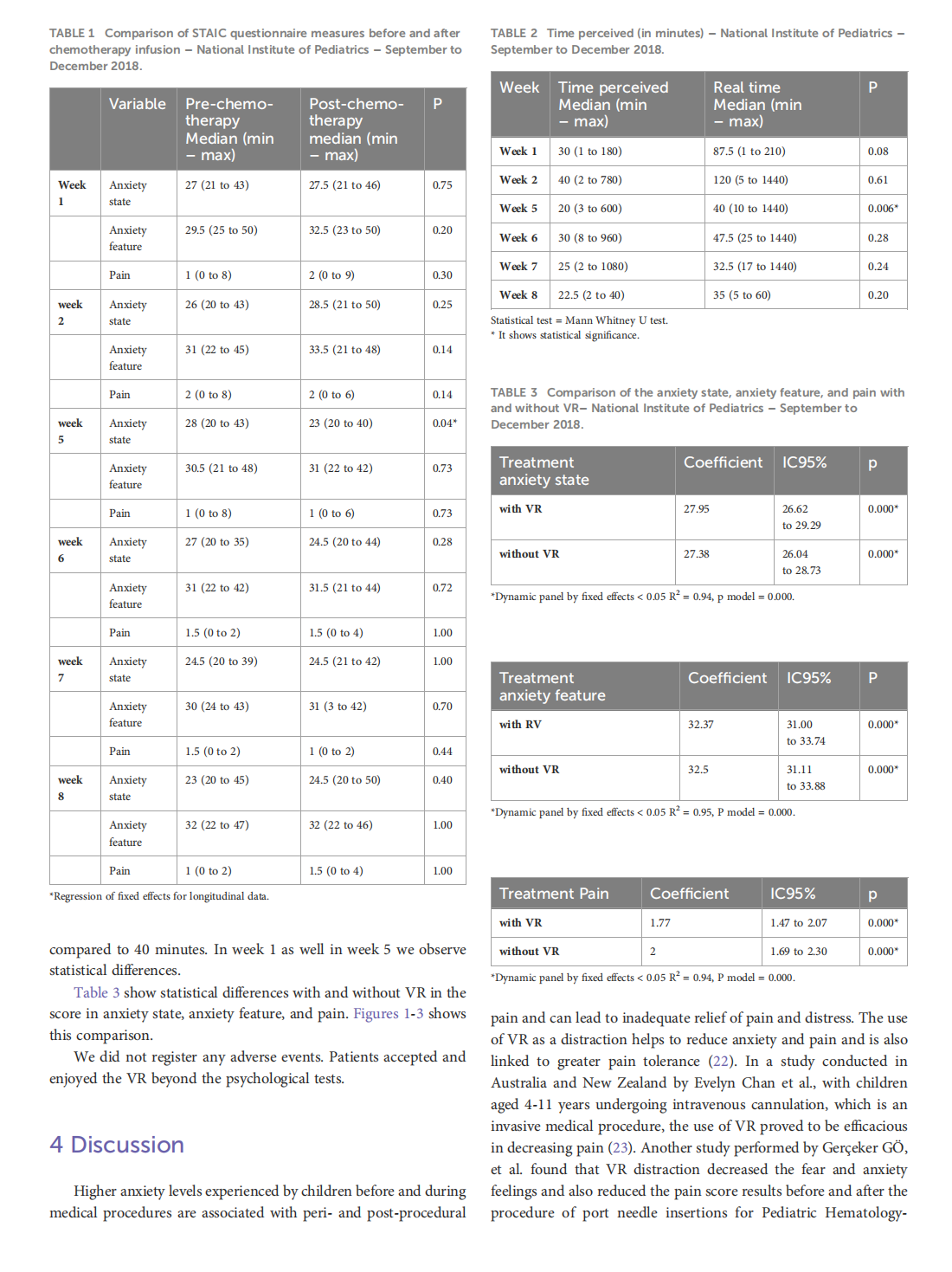
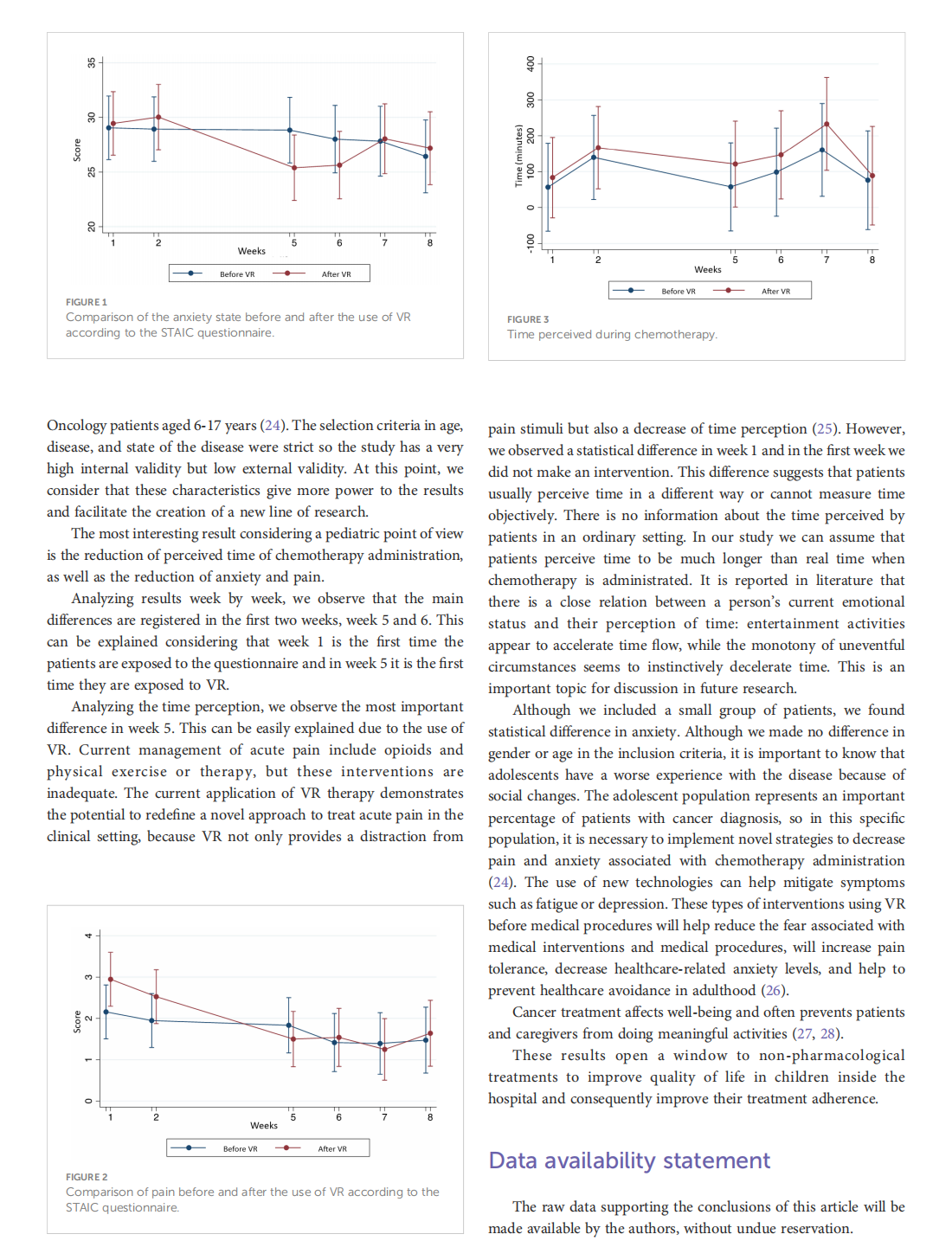

This article is excerpted from the《Frontiers in Oncology》by Wound World

- 星期四, 05 2月 2026
Research progress of electrochemical oxidation and self-action of electric field for medical wastewater treatment

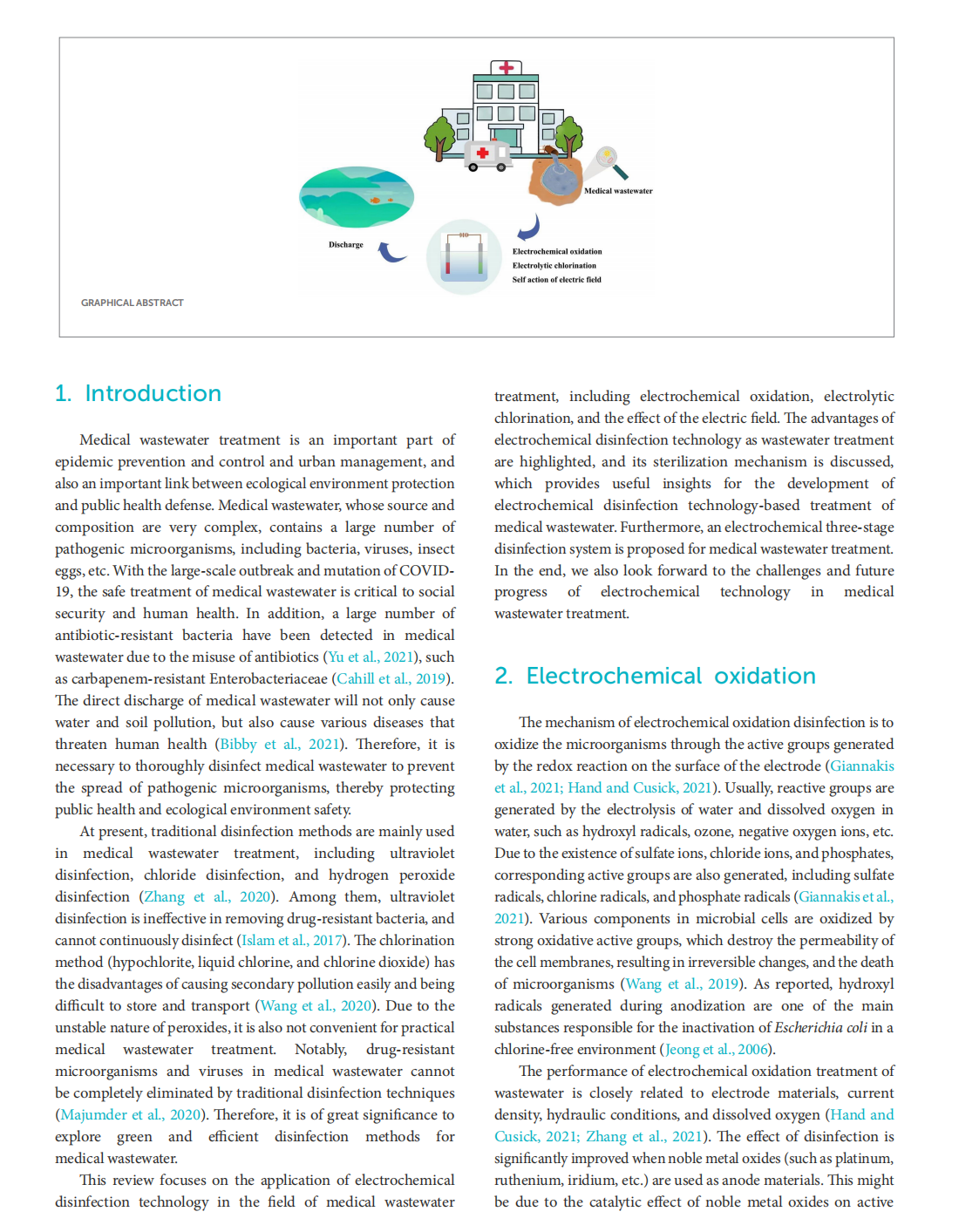




This article is excerpted from the《Frontiers in Microbiology》by Wound World


