文献精选

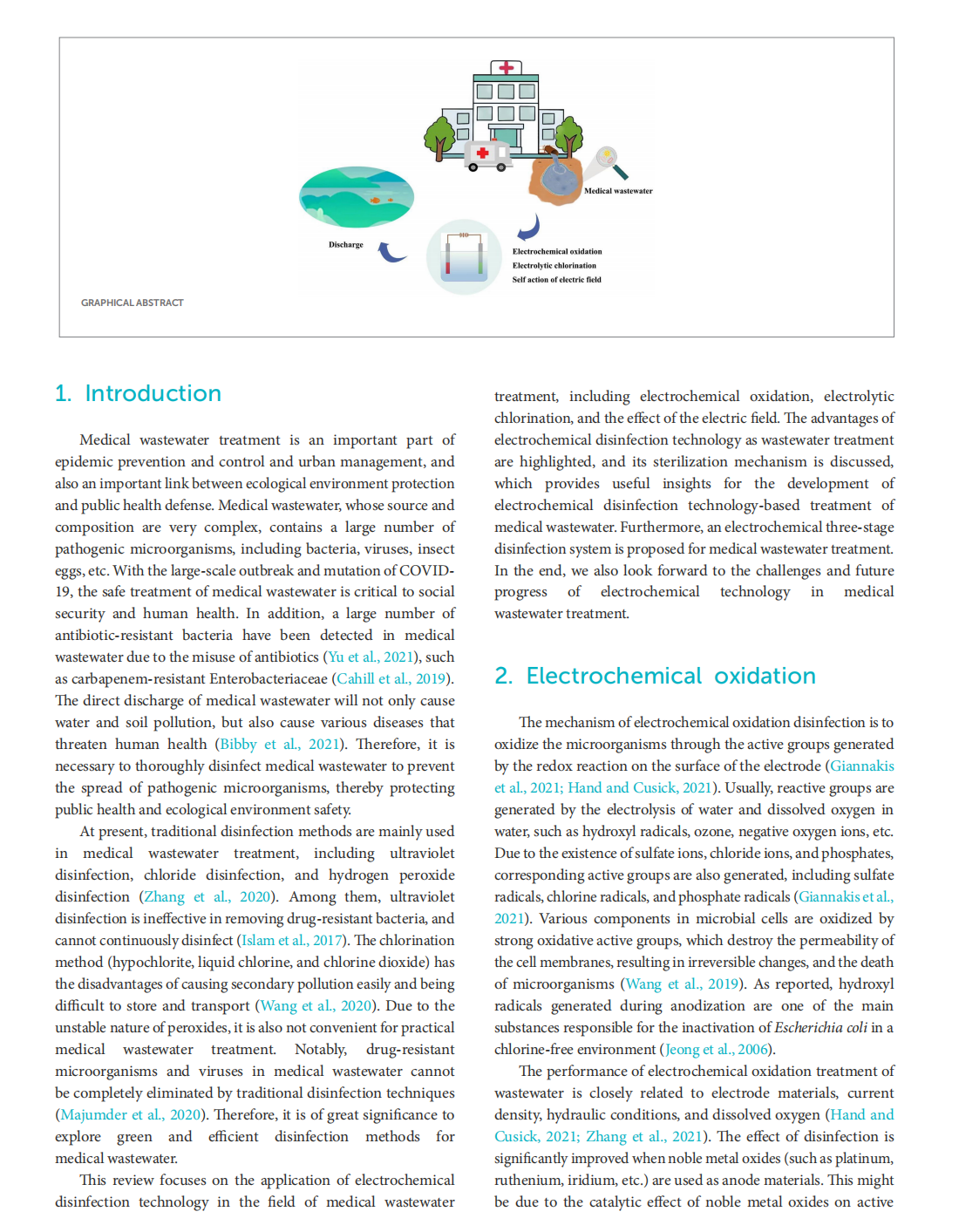




This article is excerpted from the《Frontiers in Microbiology》by Wound World


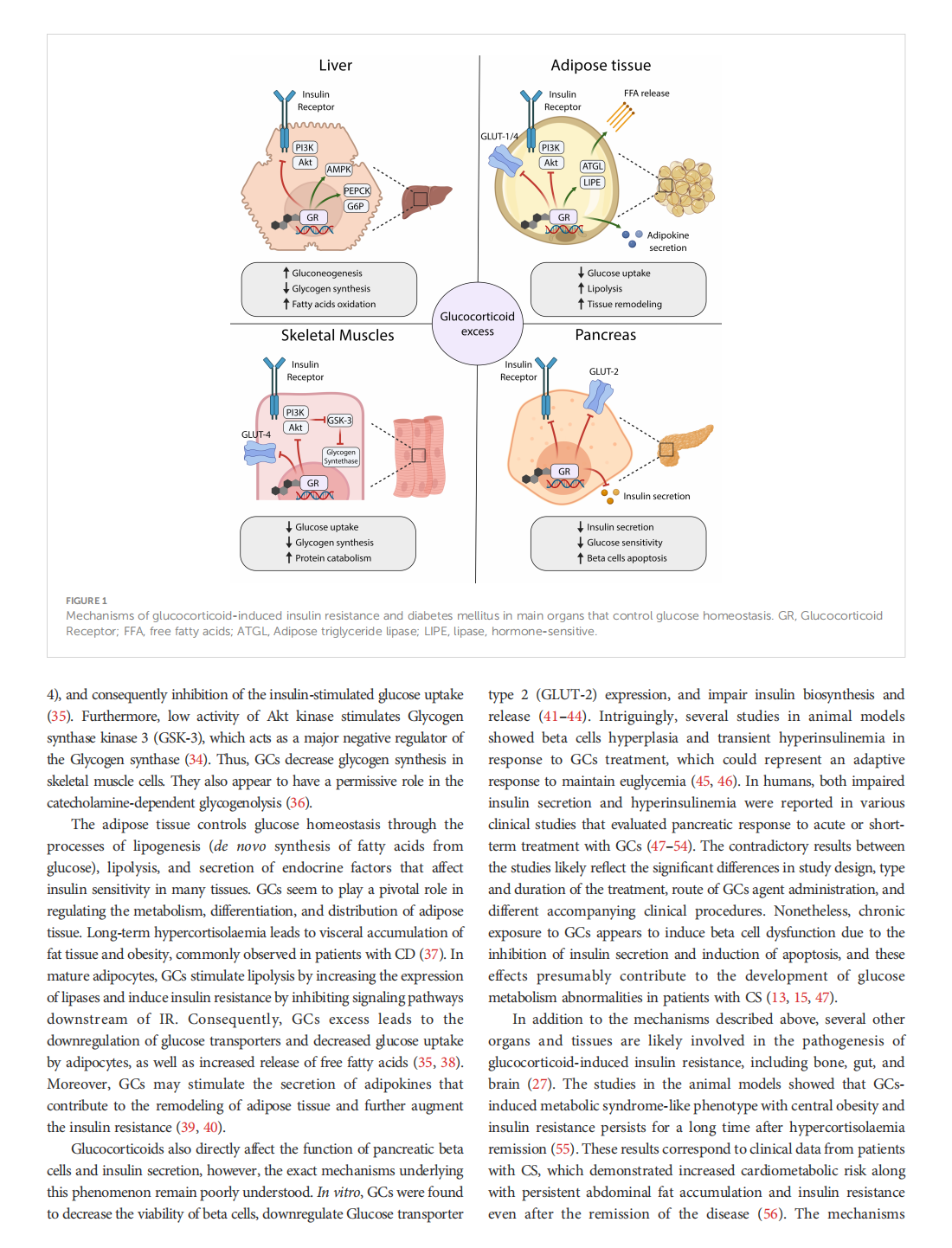







This article is excerpted from the《Frontiers in Endocrinology》by Wound World






This article is excerpted from the 《Frontiers in Oncology》 by Wound World


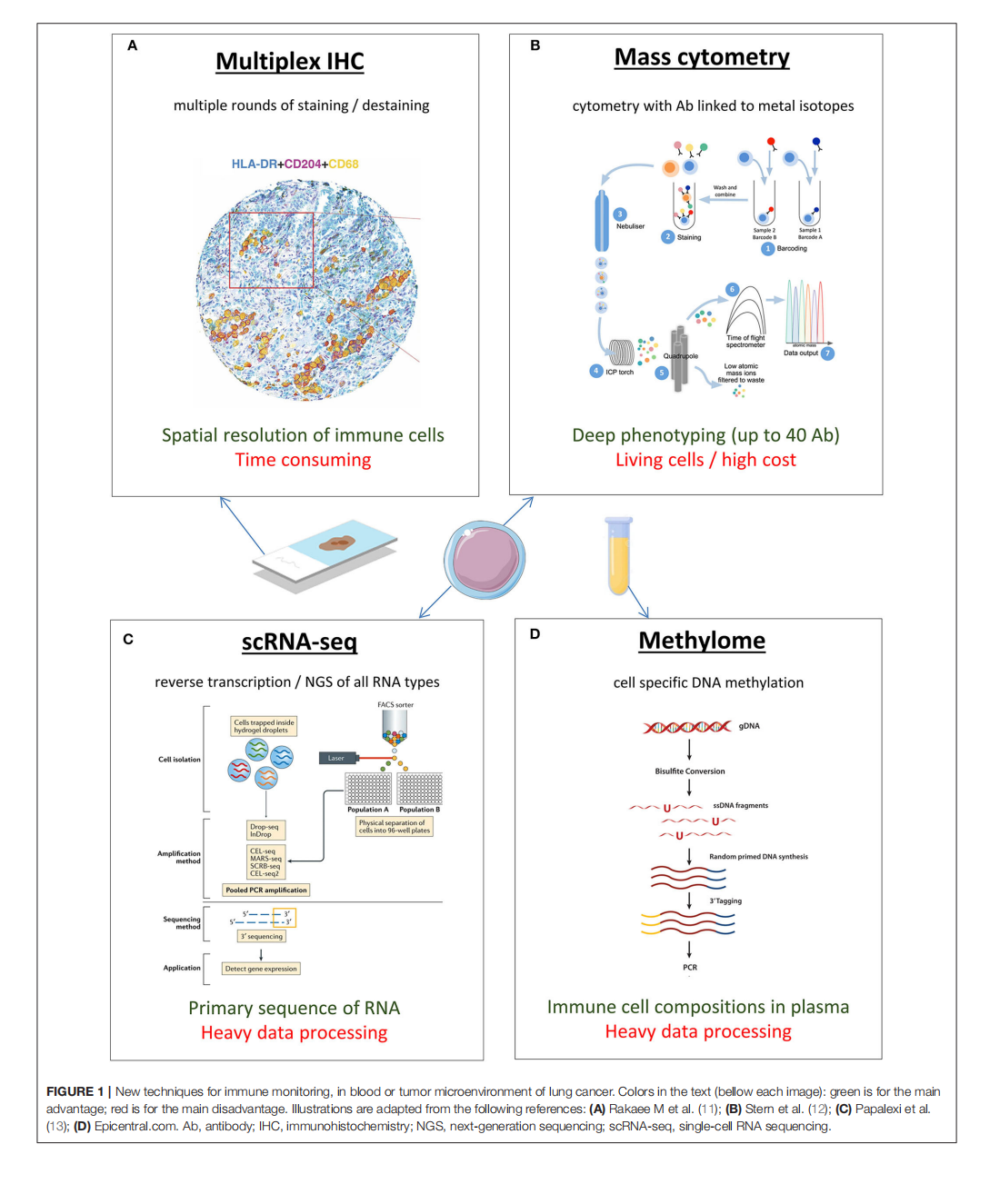

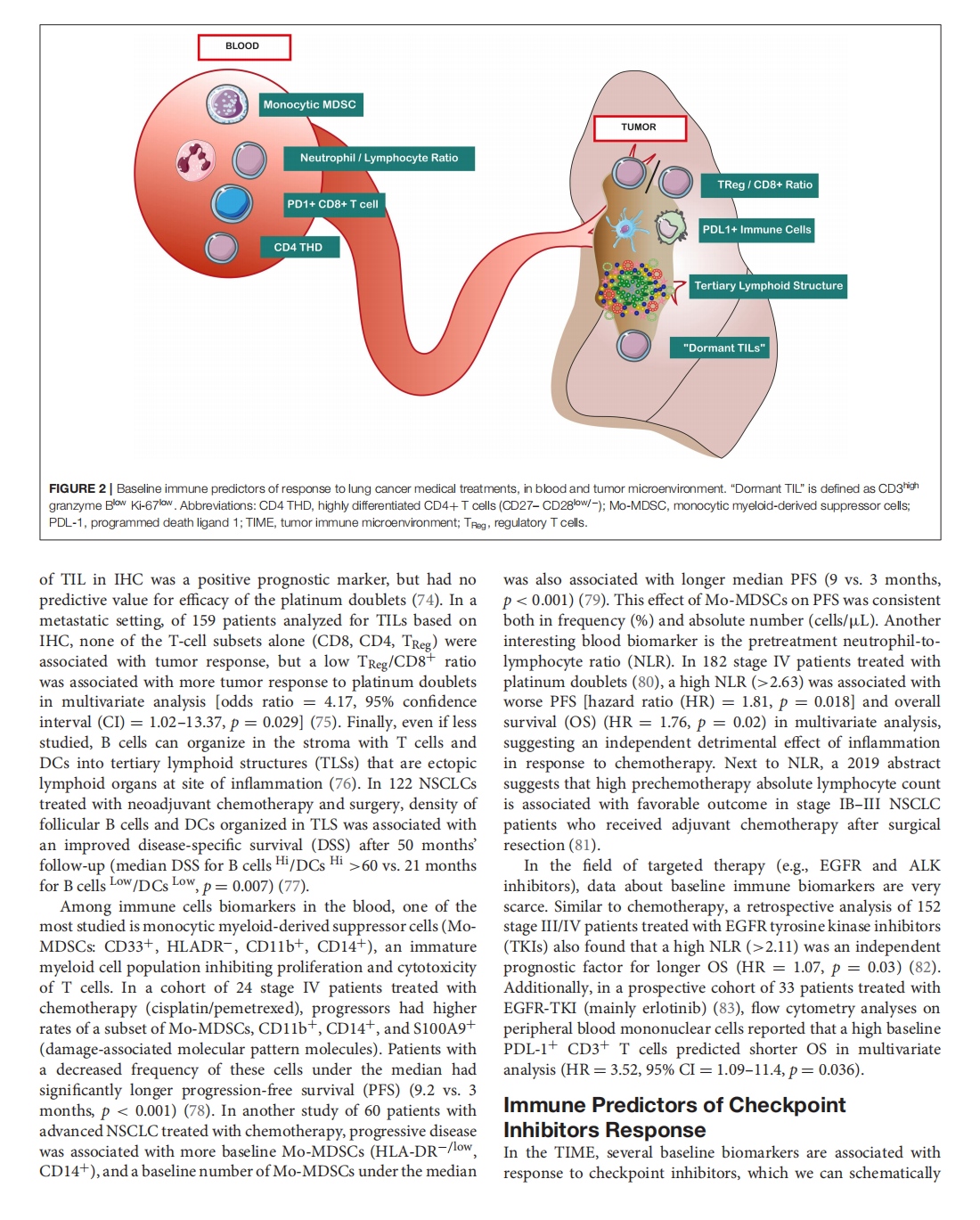





This article is excerpted from the《Frontiers in Oncology》by Wound World




