文献精选


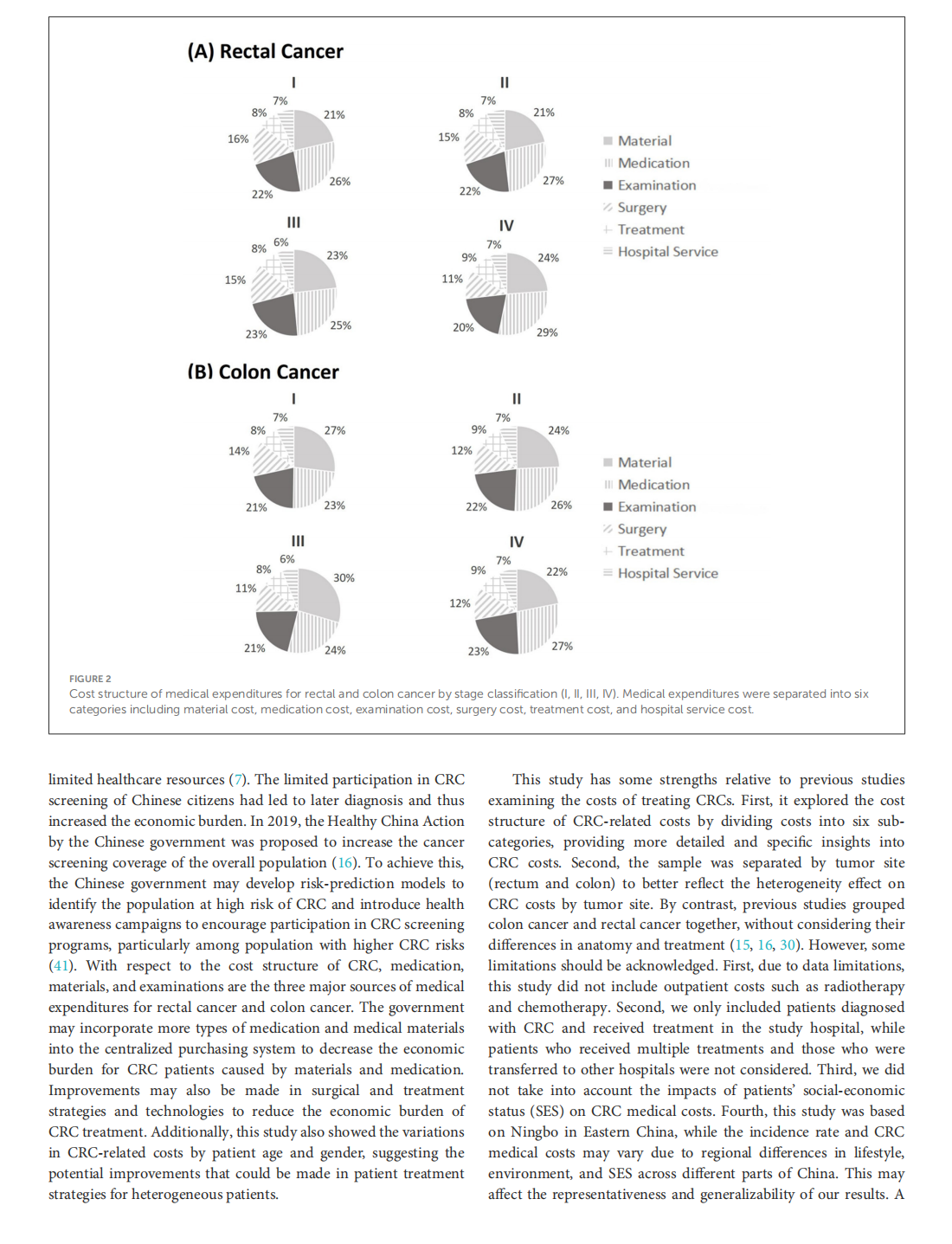


This article is excerpted from the 《Chinese Journal of Cancer》 by Wound World



















This article is excerpted from the 《Frontiers in Bioengineering and Biotechnology》 by Wound World


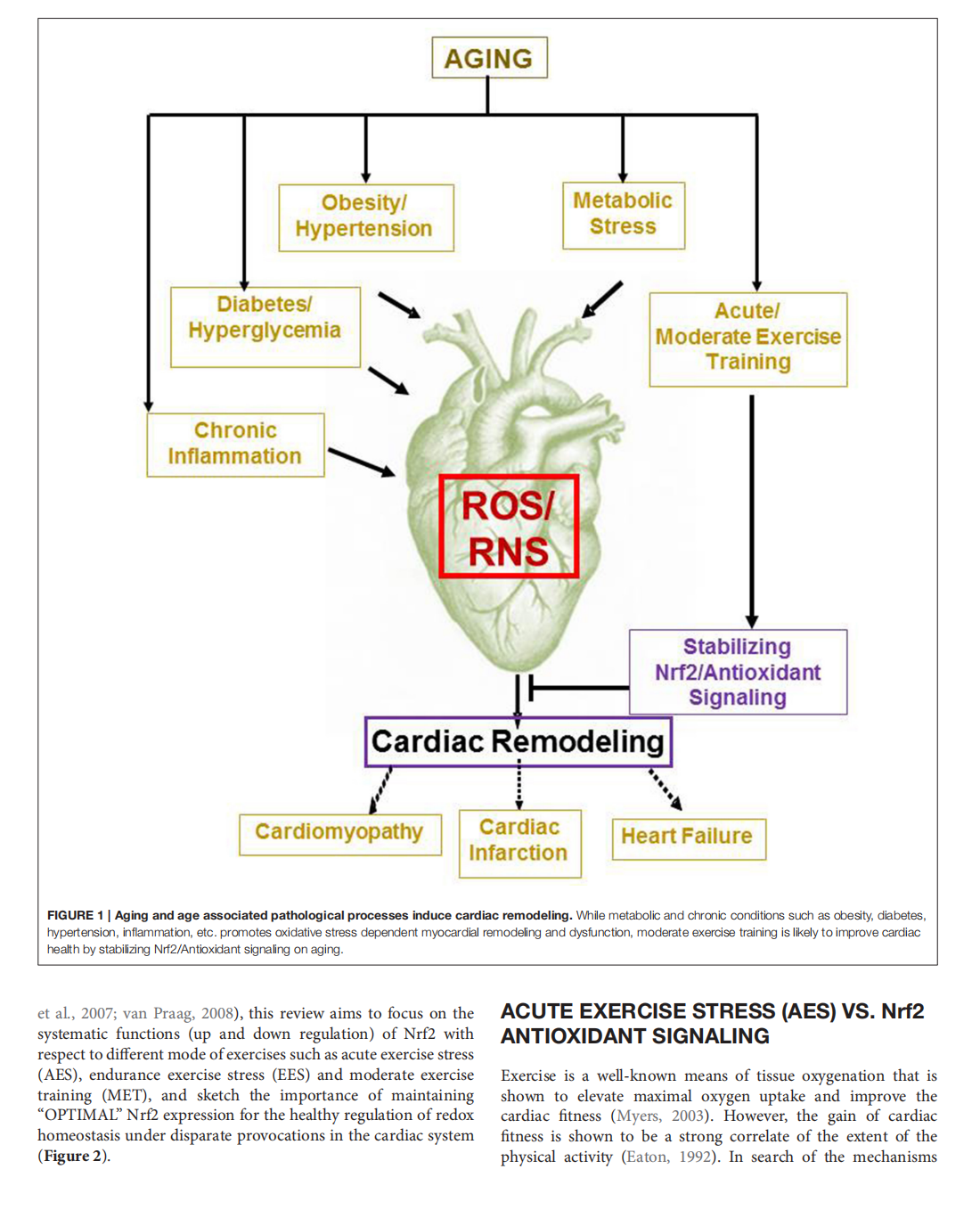
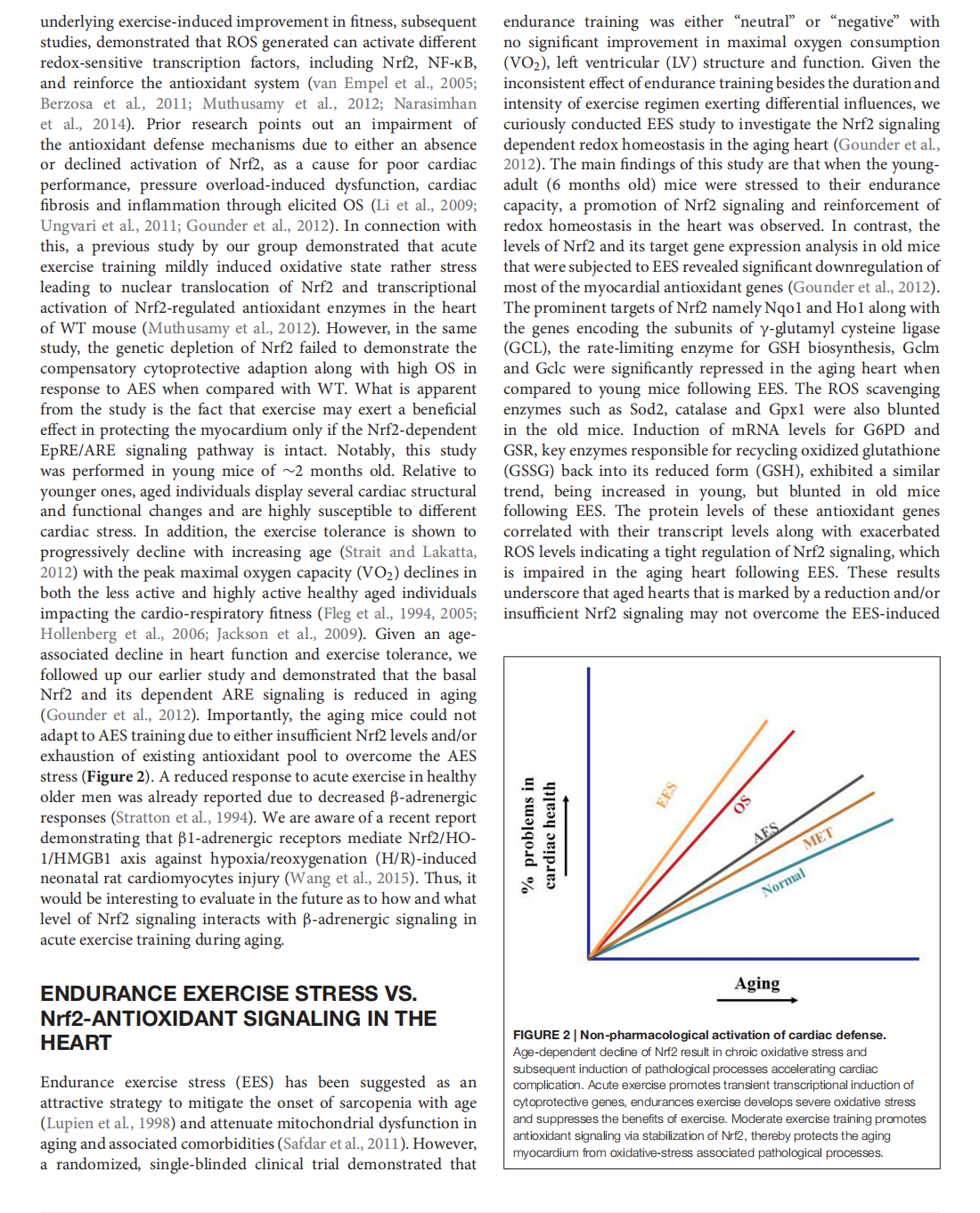



This article is excerpted from the 《Frontiers in Physiology》 by Wound World










This article is excerpted from the《Frontiers in Surgery》by Wound World




