Introduction
Keloids are dermal proliferations of fibrous tissue that most often arise at sites of cutaneous injury and have significant impact on quality of life. Although keloids are seen in all populations, the highest prevalence is in people of color with an estimated incidence of 4–16% [1, 2]. These growths represent the most robust form of abnormal wound healing, presenting as raised, firm lesions that extend beyond the margins of original injury [2]. Several etiological factors have been proposed, including genetic and hormonal influences [3]. Increased wound tension has also been associated with keloid formation, although body locations with limited tension such as the earlobe are similarly affected [4].
Multiple hypotheses have been proposed for keloid formation. Tough the pathogenesis of keloids is not fully understood, it likely involves the dysregulation of complex inflammatory pathways [5]. Proinflammatory cytokines IL-6 and -8 have been shown to increase scarring, while similarly, a decrease anti-inflammatory IL-10 increases scarring [6]. Keloidal fibroblasts and inflammatory cells may drive keloid formation by dysregulation of normal collagen turnover. Keloids are characterized by an increased ratio of type 1 to type 3 collagen deposition in a haphazard pattern with increased fibroblast proliferation rates and increased sensitivity to growth factors [6, 7]. Differences in growth factor production could be due to epithelial-mesenchymal interactions, retention of fetal proliferative pathways, or the hypoxic keloidal tissue environment. Tissue tension has also been implicated as mechanical tension is a driver of fibroblast activity and formation of collagen. Certain inherited human leukocyte antigen subtypes have been associated with keloids, suggesting an abnormal immune response to dermal injury as a cause of keloids. Lastly, dermal injury causing an immune response to sebum, leading to cytokine release stimulating mast cell infiltration and fibroblast activity, has been suggested given the predilection for keloids to form in sites of increased density of pilosebaceous units [7].
Keloids pose a significant functional and cosmetic burden. They are often pruritic or painful [8]. Additionally, they can introduce tension in adjacent tissue and cause restrictions in normal movement. The psychosocial effects of developing disfiguring scars have also been repeatedly demonstrated [9, 10]. Unfortunately, keloids do not regress spontaneously and are often refractory to Current treatment options include intralesional and topical therapies, surgical interventions, radiation, and laser based therapies [11–13]. Intralesional corticosteroids are a mainstay of treatment, although other injectables include bleomycin, 5-fourouracil, botulinum toxin type A, verapamil, avotermin, IL-10, mannose-6-phosphate, and insulin. Topical therapies include imiquimod and mitomycin C. Surgical excisions are often paired with a combination of these adjuvant pharmacotherapies, and there is ongoing innovation in keloid excision and wound closure technique. Radiation therapies include external-beam radiation and interstitial brachytherapy administered at low- or high-dose rates [13]. Pulsed dye laser (PDL), cryotherapy, and pressure dressings are often utilized, as well as overthe-counter silicone sheets and topical vitamin E creams. Despite the myriad of proposed treatment options, keloids continue to pose a therapeutic challenge, and an updated body of evidence-based recommendations to guide disease management is lacking.
Objective
The objectives of this systematic review were to examine the evidence from the past decade for the treatment of keloids, determine the efficacy and limitations, and recommend areas for improvement.
Methods
This systematic review of the relevant literature on keloid treatments was conducted according to methods outlined in the Cochrane Handbook and reported according to the recommendations from the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines.
Search strategy
A medical librarian (C. M.) created the search strategy to investigate therapies for keloid treatment published in English between the years 2010 and 2020. On November 24, 2020, searches were conducted on PubMed (National Library of Medicine), Embase (Elsevier), and Cochrane Library (Wiley) using keywords and subject headings related to “keloid” and “treatment.” The full search strategy is available at https://doi.org/10. 18131/g3-b39v-s030.
Inclusion criteria
Articles were included if they were peer-reviewed, had a prospective study design (including non-randomized interventional studies and randomized controlled trials), reported on clinical outcomes of keloid treatments, and were published in English between January 1, 2010, and the day searches were conducted (November 24, 2020).
Screening and study selection
Studies from the search result were downloaded into an EndNote database. Two reviewers independently screened titles and abstracts of all obtained studies, ensuring studies met the inclusion criteria. Any disagreements were then consulted with a third independent reviewer. Full texts of studies that were included by title and abstract screening were further reviewed, again independently by the two reviewers. Any disagreements were also consulted with a third independent reviewer as needed.
Risk‑of‑bias assessment
Risk of bias for studies that were classified as randomized controlled trials was evaluated with the RoB 2: a revised Cochrane risk-of-bias tool for randomized trials [14]. Five categories of bias — randomization process, deviations from intended interventions, missing outcome data, measurement of the outcomes, and the selection of reported outcomes — were assessed using the RoB 2 algorithm and classified as low risk, some concerns, high risk, or no information.
For studies that were non-randomized interventional trials, the risk of bias in non-randomized studies of interventions (ROBINS-I) assessment tool was used to evaluate the risk of bias in seven categories: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, outcome measurement, and selective reporting [15]. The ROBINS-I guide was used to grade each category as low risk, moderate risk, serious risk, or no
Figures of the risk-of-bias results were created using the risk-of-bias VISualization (robvis) online tool [16].
Data extraction
Two reviewers independently extracted data from the studies in the EndNote database. The following data were extracted as follows:
1. Publication details: Authors and date of publication
2. Study design: I.e., randomized control trial, single- or double-blind, split-scar study
3. Participants: Number of participants and demographics
4. Type of treatment or intervention
5. Outcomes including subject- and physician-reported responses to treatment, objective measures of treatment, recurrence rates, follow-up time, and adverse
Data synthesis
We were not able to pool data from multiple studies given the heterogeneity of measurements used for quantifying outcomes. Data extracted from eligible studies were analyzed using a narrative approach. Tis synthesis aimed to provide an evidence-based review of all prospective data regarding keloid treatments and outcomes in the last decade.
Results
Overview
There were 3462 articles included in the literature search. Screening of titles and abstracts yielded 440 articles for full-text evaluation, of which 108 were included, 305 were excluded, and 27 did not have full texts available to obtain (Fig. 1). Exclusion reasons included retrospective study design (80), wrong publication type (50), wrong study design (45), nonclinical outcome (14), wrong population (14), hypertrophic scar (96), and foreign language (6).

The total sample size was 4552 subjects (range of 6–240). The follow-up times varied from 4 weeks to 10 years. Tere were 37 randomized studies, 4 split scar studies, and 1 placebo-controlled studies.
Risk of bias in the 37 randomized controlled trials was low overall throughout the domains assessed in RoB 2 (Fig. 2). The measurement of outcomes domain had the highest proportion of studies with some concerns of bias, mainly due to lack of evaluator blinding and differences in timeframe of follow-up amongst the interventions (see Additional file 1 for the RoB 2 assessment for each study). Similarly, majority of non-randomized interventional studies were rated as low or moderate risk of bias with the ROBINS-I algorithm (Fig. 3). Only 4 out of the 71 non-randomized interventional studies had some components of serious risk of bias (see Additional file 2 for the ROBINS-I assessment for each study).


Corticosteroids
Intralesional corticosteroids are the most commonly used nonsurgical treatment for keloids (Table 1). Intralesional triamcinolone acetonide (IL TAC) 10–40 mg/ml is most ubiquitous and induces keloid regression through a variety of proposed mechanisms including suppression of dermal inflammation, reduction of oxygen delivery to the wound bed via vasoconstriction, and antimitotic activity in keratinocytes and fibroblasts [17]. In review of 19 articles, there was unanimous clinical improvement in keloids with intralesional corticosteroid treatment. However, the degree of improvement and its relationship with treatment characteristics such as dosage, frequency, and timing of injections were variable [18–36].
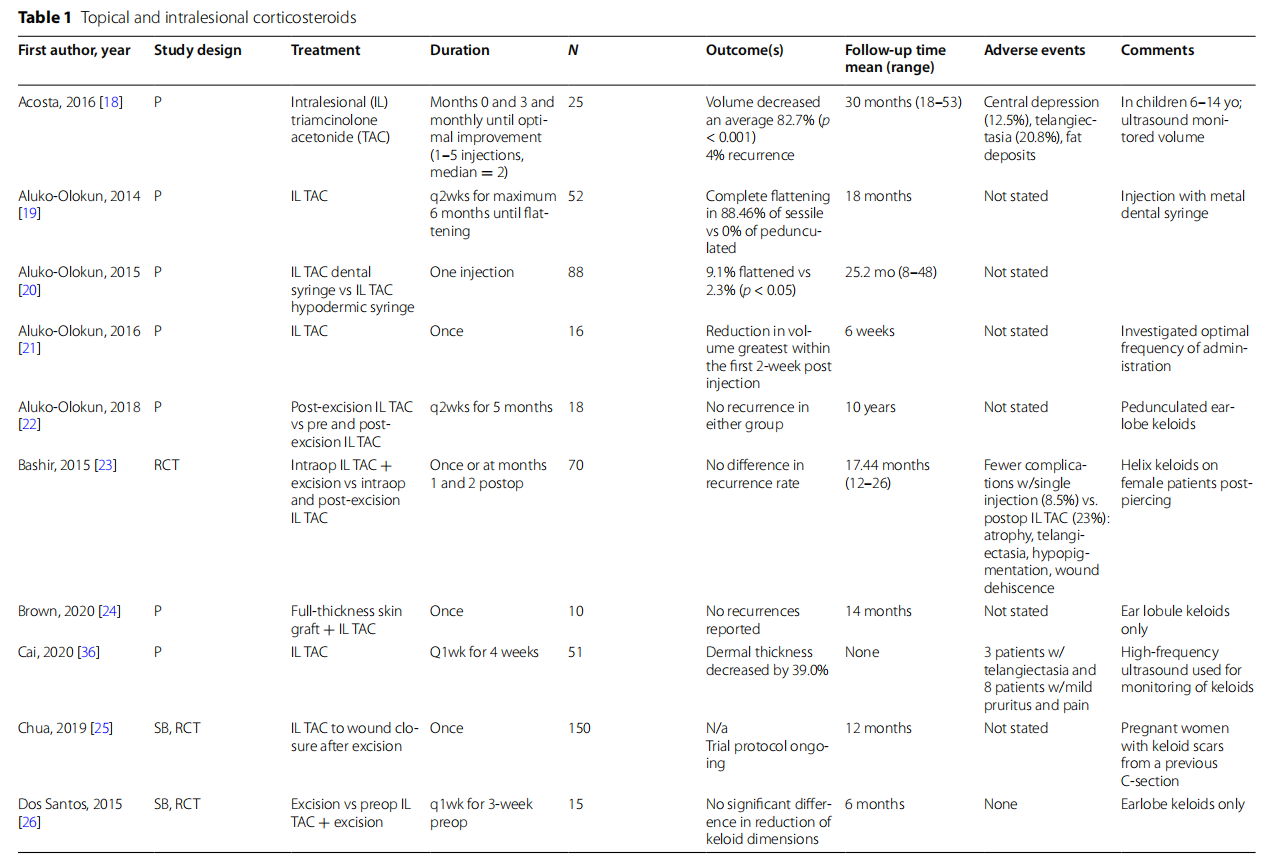

In terms of dosing, 20–40 mg/ml of triamcinolone acetonide was most commonly investigated (8 of16 studies). Notably, a study by Huu et al. compared IL TAC 7.5 and 14 mg/cm2 and found a larger proportion of “good” and “quite good” results in the smaller dosage group; however, the size and characteristics of the studied keloids were not specified [28]. Frequency of treatments ranged from single injections to weekly and monthly injections. Aluko-Okun et al. (2016) studied optimal TAC dosing and observed the greatest reduction in keloid volume with 2-week treatment intervals [21].
Intralesional TAC was combined with surgical excisions in several studies with mixed results. Tripoli et al. reported no recurrences in subjects treated with two dosages of TAC after radial excision at their 2-year follow-up [35]. This is compared to the 9 controls who were excised without TAC and demonstrated a 67% recurrence rate. However, Dos Santos et al. compared excision +/− 3 weeks of preoperative 20-mg triamcinolone hex acetonide and found no significant difference in keloid dimensions at 6-month follow-up [26]. Bashir studied intraoperative TAC vs. intraoperative and postoperative TAC in 70 subjects and found no significant difference between the two groups [23]. Finally, when IL TAC 20 mg/ml was combined with intralesional radiofrequency in a cohort of 60 subjects, Kaushal et al. reported fewer recurrences at 6 months compared to IL TAC alone [29].
In addition to treatment parameters, keloid response is likely influenced by lesion characteristics. Aluko-Olokun et al. (2014) compared response of sessile vs. pedunculated lesions to TAC 10 mg and found a lack of response by pedunculated lesions compared to fattening of 23 of the 26 treated sessile lesions [19].
While topical steroids are less commonly used in the treatment of keloids, Nor et al. compared IL TAC 40 mg/ml monthly for 3 sessions to daily topical clobetasol propionate 0.05% cream under occlusion with silicone dressing [30]. There was no significant difference in reduction in keloid size; however, topical treatment resulted in significantly fewer adverse effects.
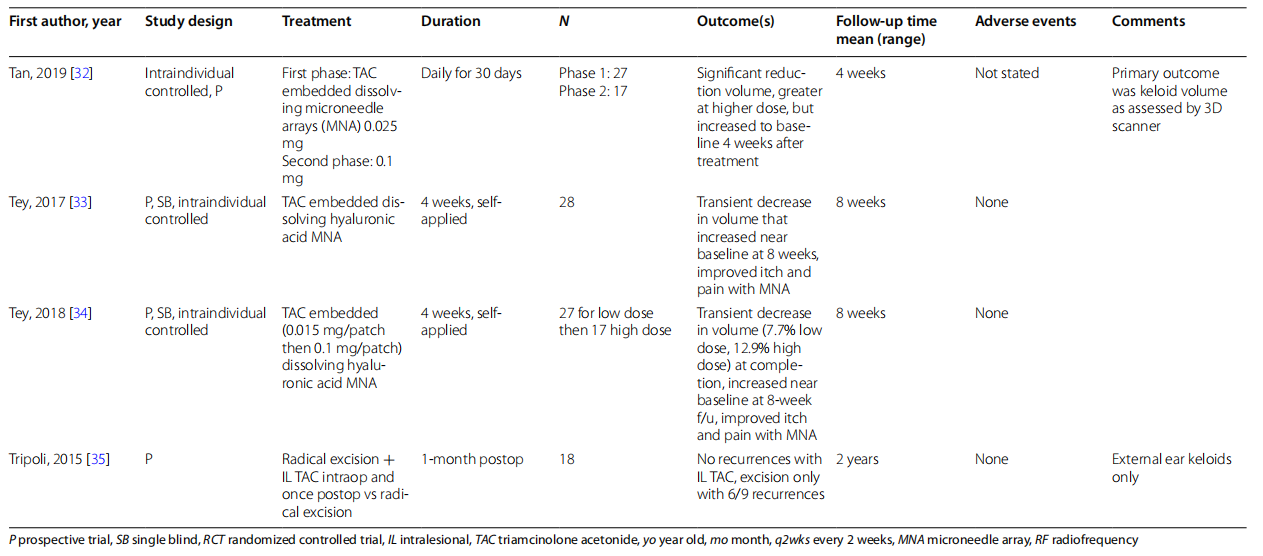
Finally, there is innovation in TAC drug delivery modalities, including a metal syringe and drug embedded microneedles. The metal syringe was proposed by Aluko-Olokun et al. as a new delivery system to address the issues of syringe failure and inadequate drug delivery to firm lesions [20]. Dissolving microneedles are self-administered once a month, empowering patients in their own care and reducing the inconvenience of frequent office visits. Initial studies suggest that these alternate delivery methods yield superior results compared to traditional plastic syringes. However, for TAC embedded microneedle arrays (MNAs), the volume decrease seems to be transient and not a durable response [33, 34].
Cryotherapy
Cryotherapy or cryosurgery is a long-standing technique which relies on the reduction of temperature to cause irreversible cellular damage (Table 2). For treatment of keloids, studies have shown that cryotherapy transitions the keloidal fibroblasts towards a normal fibroblastic phenotype, increasing the ratio of type 3 to type 1 collagen in vitro [37, 38]. An additional advantage is that the decellularized matrix is left as a scaffold, possibly preventing recurrence. Cryosurgery alone has been shown to fatten keloids [39]. Intralesional verapamil, cryosurgery alone, or cryosurgery with intralesional TAC or verapamil all showed significant (p < 0.001) improvement in all VSS variables with no difference from cryosurgery with IL TAC [40]. Similarly, Fraccalvieri showed that cryosurgery alone or in combination with shave removal led to a majority of subjects (83% of 76 subjects) experiencing a 75–82% decrease in keloid height [41]. A smaller study of 12 subjects showed that a combination of shave removal, cryosurgery, and IL TAC had only 1 recurrence with 75% of subjects seeing a significant reduction in thickness [42]. Additionally, a combination of surgical excision, cryosurgery, and platelet-rich plasma (PRP) led to 70% of the 50 subjects observing improvement in keloid height and a recurrence 6 lesions after 7 months of follow-up [43].

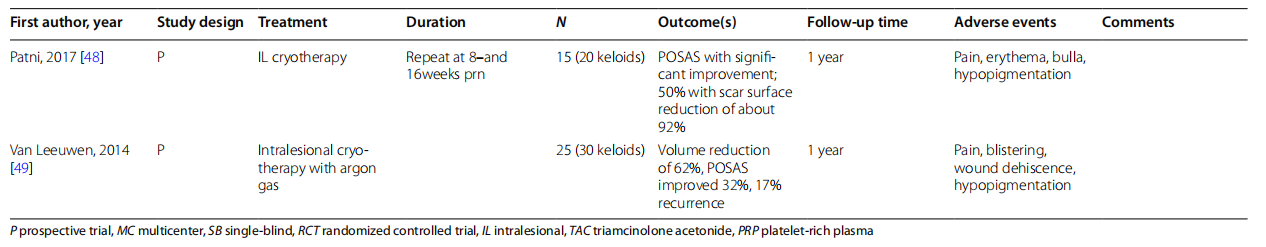
Intralesional cryotherapy was first introduced in 1993 [50]. Patni et al. showed that with up to three sessions of intralesional cryotherapy, subjects saw a significant improvement of POSAS, and 50% of subjects saw a scar surface reduction of about 92% [48]. Additional recent investigation in the field of keloid treatment has compared intralesional cryotherapy to open spray cryotherapy. Mourad et al. and Abdel-Meguid et al. both showed that intralesional cryotherapy improved clinical appearance of keloids [44, 47]. However, a randomized trial by Bijlard et al. was terminated prematurely due to intralesional cryotherapy having inferior results to excision and IL TAC for primary keloids and excision and RT for resistant keloids [46]. A new innovation to intralesional cryotherapy is the use of argon in place of liquid nitrogen. The benefit is more controlled and accurate freezing and has a well-established history of use within the field of oncology. Van Leeuwen et al. - showed a volume reduction of 62% [49]. However, further comparative studies will likely be required for such a technique to become more widely adopted.
Intralesional injection
Many non-corticosteroid intralesional injections and combination treatments have been studied for keloid - treatment including verapamil hydrochloride, 5-fuo rouracil (5-FU), bleomycin, botulinum toxin A (BTA), hyaluronidase, and platelet-rich plasma (PRP) (Table 3). - In many cases, TAC was used as the control group treatment when investigating these other agents.


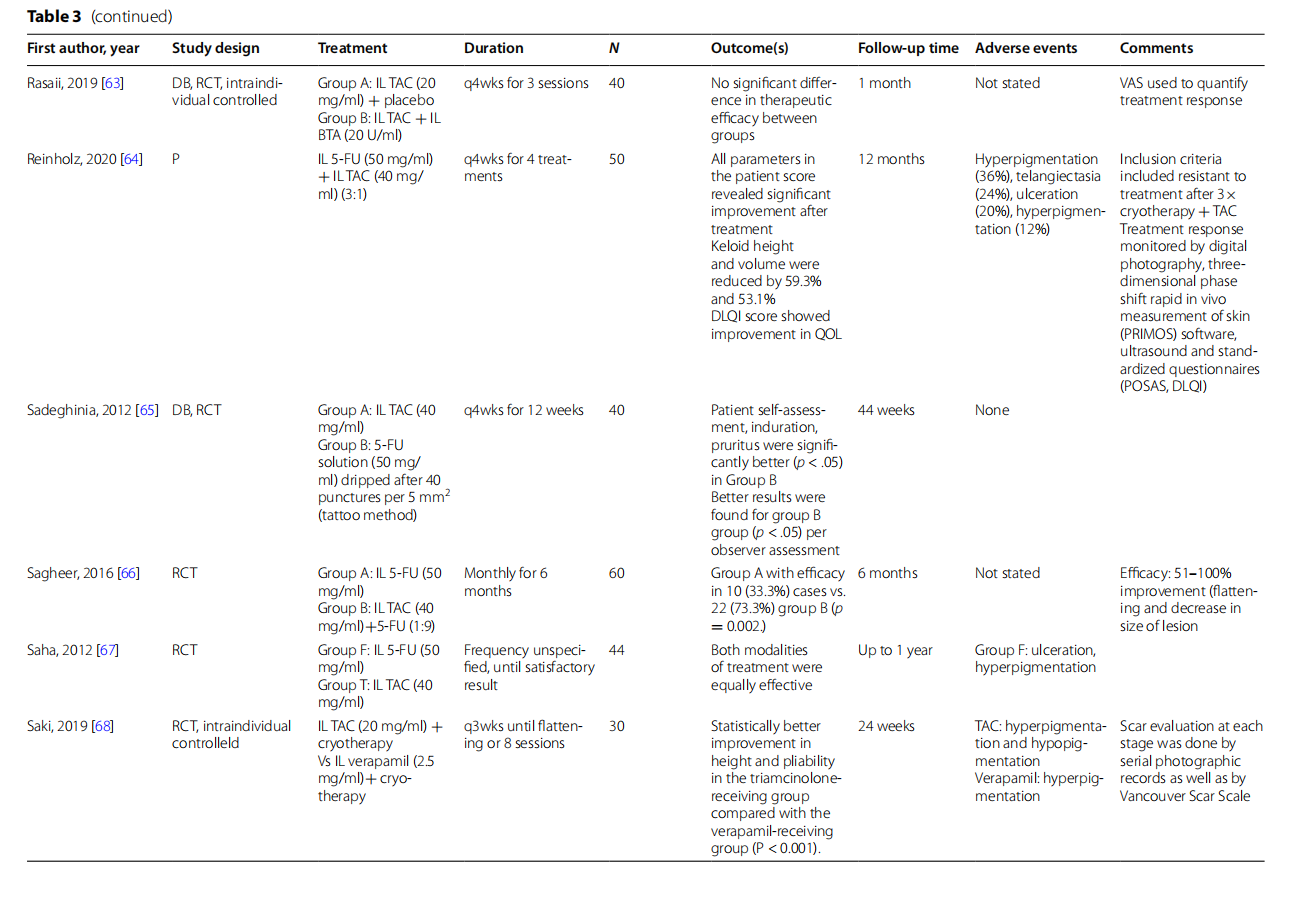
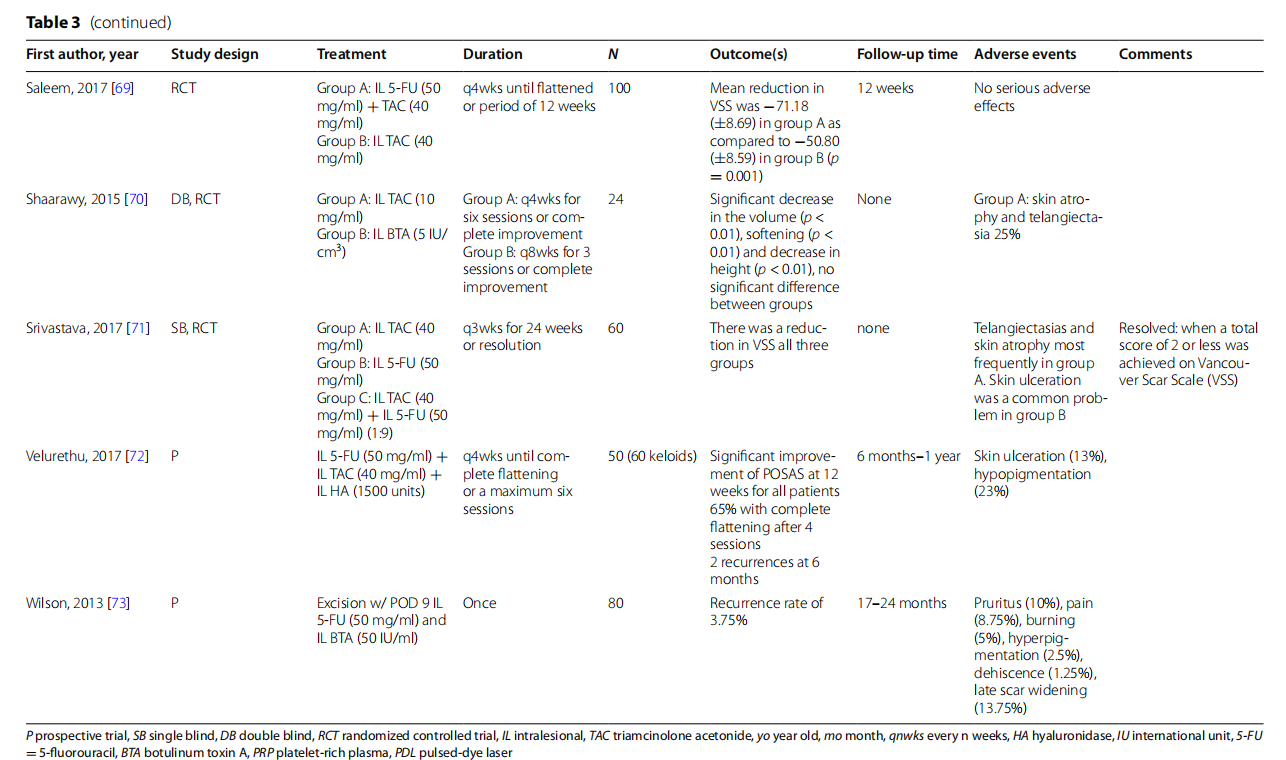
Verapamil is a calcium channel blocker that sup presses extracellular matrix molecules formation and promotes collagen breakdown. It is commonly used in the concentration of 2.5 mg/ml when treating keloids. In several noncontrolled studies, verapamil treatment alone or in combination with keloidectomy or pulse dye - laser (PDL) resulted in decreased VSS scores and positive clinical response [51, 55, 61]. However, intralesional verapamil was inferior when compared to IL TAC. In a double-blinded controlled trial comparing 4 monthly doses of verapamil to identically scheduled TAC 5 mg/ml - in 14 keloid lesions, there was significantly higher recur rence rates at 12-month follow-up with a hazard ratio for recurrence of 8.44 ( 95% CI 1.62–44.05) [54]. In their intraindividual study, Saki et al. compared verapamil + cryotherapy to TAC 20 mg/ml + cryotherapy in opposite ends of the same lesion (a split scar study); results showed - statistically greater reduction in height and improved pliability in the TAC group [68].
Bleomycin is an antineoplastic agent that causes necrosis of fibroblasts. Two studies investigated bleomycin and demonstrated its utility in keloid treatment [59, 74]. Khan et al. most robustly showed this effect in 164 keloids: 6 - doses of monthly 1.5 IU/m was more effective than identically scheduled TAC 40 mg/ml, achieving 50% reduction in the POSAS score from baseline. This difference was independent of age, gender, Fitzpatrick skin type, the duration of keloids, or baseline POSAS score [59].
The antimetabolite 5-FU inhibits fibroblast proliferation through disruption of DNA replication. 5-FU is used independently and in combination with other treatments, most commonly IL TAC. Saha et al. compared 5-FU with TAC in 44 subjects and showed both were equally effective [67]. Ali et al., in a randomized controlled trial comparing 50 mg/ml 5-FU alone with combination 5-FU 50 mg/ml (0.9 ml) + 40 mg/ml TAC (0.1 ml), showed that reduction of mean keloid height after treatment was significantly greater in the combination group (p = 0.0008) [53]. Saleem et al. similarly showed a combination of TAC+5-FU had significantly greater improvement in VSS than TAC alone in 100 subjects [ 69]. Sagheer et al. demonstrated similar superiority of combination TAC 40 mg/dl (0.1 ml) and 5-FU 50 mg/ml compared to 5-FU alone [66]. Notably, adverse effects were not reported in either study; however, in another noncontrolled study, Reinholz et al. demonstrated local adverse effects in > - 90% of their subjects, including hyperpigmentation, telangiectasia, and ulceration [64]. Srivastava et al. compared TAC vs. 5-FU vs. TAC + 5-FU and showed all improved VSS scores compared to baseline in 60 subjects [75]. Finally, Sadeghinia et al. compared intralesional TAC 40 mg/ml to 5-FU applied by a unique tattoo method [65]. In the latter group, 5-FU 50 mg/ml solution of the lesions. Subsequently, was dripped on each 1 cm2 were made followed by a second 40 punctures per 5 mm2 - round of 5-FU drip application. Tis methodology theo retically allows for deeper and more even penetration of - the drug and resulted in significantly decreased induration and pruritus and improved observer assessment by - a blinded dermatologist with respect to overall improvement on a 5-point scale.
Botulinum toxin A (BTA) is a neurotoxin known for its paralytic effects. Its utility in keloid treatment may be related to reduction of muscular tension at wound sites and direct fibroblast regulation. No significant difference - was found in 2 double-blinded controlled trials comparing 5 IU/cm to TAC 10 mg/ml and BTA 20 μ/ml to TAC 3 20mg/ml, respectively [63, 70]. Interestingly, in a head-to-head comparison between 5-FU 50 mg/ml and BTA , Ismail et al. showed significantly greater fat- 2.5 U/cm3.Ismail et al. showed significantly flattening by BTA (p = 0.04) [58]. As a combination therapy, Gamil et al. showed significantly ( p = 0.0001) reduced keloid surface area in 24 keloids treated with intralesional BTA and TAC compared to 26 subjects treated with TAC or BTA alone [56].
The enzyme hyaluronidase catalyzes the breakdown of the mucopolysaccharide hyaluronic acid. Although it - has been studied in the treatment of keloids, its mechanism of action is not clearly understood. Aggarwal et al. - showed that TAC + 1500 IU/ml hyaluronidase had similar clinical efficacy compared to triamcinolone alone but fewer side effects (18.75% subjects developed atrophy with combination in comparison with 31.25% subjects with triamcinolone alone, p < 0.001, chi-square test) [52]. The author highlights that in the combination group, - the TAC dosage was effectively halved, suggesting a synergistic effect of TAC and hyaluronidase combination treatment. Velurethu et al. showed a combination of intralesional 5-FU, TAC, and hyaluronidase every 4 weeks for 50 subjects with 60 keloids led to fattening in 65% and > 90% reduction in scar volume in 35% of keloids after 4 sessions [72]. Only two recurrences were observed at follow-up after 6 months.
PRP is autologous platelet concentrate that is used in a variety of conditions to promote wound healing, decrease pain, and combat inflammation. In an RCT comparing- gold standard IL TAC 20 mg/ml every 3 weeks for 4 sessions to identically scheduled IL TAC followed by 1 injection of PRP, the latter was shown to have superior keloid response and fewer adverse effects [57].
In combination with keloid excision, intralesional treatment with the previous therapeutics is used to decrease recurrence rates. Khare et al. treated the wound bed and margin with 5-FU after excision for 28 subjects [60]. They - observed a recurrence rate of 3.57% in the 28 treated subjects compared with a 21.9% recurrence rate over 1 year in the 32 control subjects treated with IL TAC. Similarly, Wilson et al. treated 80 subjects with excision followed by IL 5-FU and BTA 9 days post surgery and observed - a recurrence rate of 3.75% [73]. Pruksapong et al. rand omized 25 subjects with 50 keloids to keloid excision and then IL TAC or IL BTA [62]. Subjects receiving IL BTA had significantly (p < 0.010) decreased VSS.
Light‑based therapy
Lasers
Both ablative and non-ablative lasers have been proposed for the treatment of keloids (Table 4). Ablative lasers include the erbium (Er:YAG) laser and CO2 laser, and they cause local tissue destruction by target-ing the water chromophore. Non-ablative lasers such as ND:YAG, diode lasers, and pulsed dye lasers (PDL) target melanin and/ or hemoglobin. The mechanism by which lasers treat keloids is less clear and may include local damage to lesional blood vessels or direct fibroblast suppression. While lasers can be used as independent therapy for keloids, they are also being investigated in combination with therapeutics to assist in drug delivery and penetration. In our cohort of prospective studies, CO2 lasers were the most frequently investigated, followed by erbium ablative lasers, ND:YAG, diode lasers, and finally PDL.

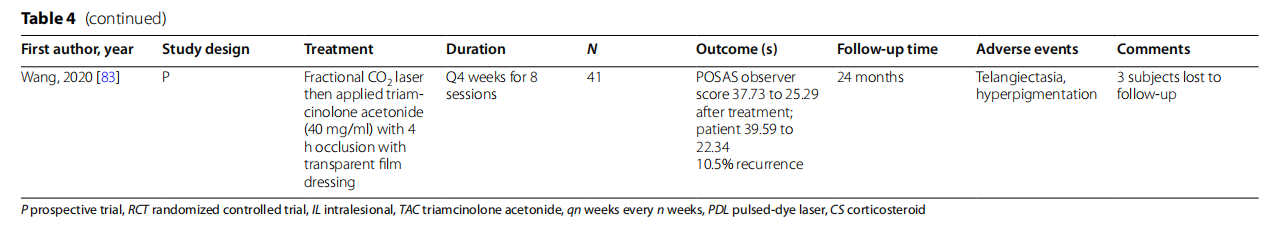
In their RCT of 60 subjects, Behera et al. found no significant difference in therapeutic response by keloids treated with 5 sessions of CO, laser compared to cryo-therapy, both in conjunction with IL TAC 40 mg/ml [78]. However, CO2 laser therapy yielded more frequent early adverse effects. A prospective study of 41 keloids treated with CO2 followed by topical TAC 40 mg/ml Q4 weeks for 8 sessions showed a recurrence rate of 10.5% at 24 months [83]. Garg et al. similarly showed a recurrence 2 with regular rate of 11.7% in subjects treated with CO2 with regular. follow-up of IL TAC in 35 treated keloids [80]. Unfortunately, there were no studies of CO laser + IL TAC compared to IL TAC alone, precluding the direct evaluation of CO2 laser treatment. Srivastava et al. compared 2 ablative laser alone compared to IL TAC 40 mg/ CO ml alone and found no significant differences between keloid response but faster improvement in the IL TAC group [71].
In a split-side controlled study, Abd EI-Deyem et al. demonstrated the superiority of fractional ablative 2940 nm Er:YAG laser-assisted delivery of betamethasone vs IL TAC 10 mg/ml alone [76]. The difference in steroid used between groups is a significant confounding variable. Conflicting results were reported in another study where no difference in clinical improvement was appreciated between keloids treated with Er:YAG laser and . IL TAC 10 mg/ml versus topical desoximetasone 0.25% ointment with 3-h occlusion [84].
A prospective study of 62 subjects showed that the addition of 1064-nm Nd:YAG to IL disprospan and IL 5-FU resulted in superior results compared to either drug alone or the two combined (78% excellent responses vs. 58% and 20%) [79]. These results make a compelling case . for Nd:YAG-assisted drug delivery. Annabathula et al. combined Nd:YAG, CO,, and PDL Q4 weeks for 5 sessions. In their 11 subjects whom completed the study, 5 showed minimal to no improvement, 4 moderate (26- 50%), improvement, and 2 > 50% improvement based on size, color, and aesthetic impression by three blinded dermatologists [77].
Kassab et al. followed clinical improvement of earlobe keloids treated with 980 nm diode followed by IL TAC 40 mg/mL Q3 weeks for a variable 2- 5 sessions [81]. While 7% of lesions shrunk at least 75% in size, the sample size was small(n= 16).
Photodynamic therapy
There is sparse but emerging evidence on the utilization of photodynamic therapy (PDT) in treating keloids and hypertrophic scars (Table 5). PDT is typically administered following the application of a photosensitizing agent such as 5-aminolaevulinic acid (ALA). While the mechanisms underlying the response of keloids to PDT are still under investigation, PDT is emerging as a potential adjunct therapeutic option for keloid treatment.

Basdew et al. conducted one of the first large- scale studies investigating the clinical use of PDT for keloid treatment,comparing surgical excision with either adjunctive interstitial brachytherapy or ALA applied to the wound bed followed by postoperative interstitial PDT using an inserted transparent catheter with a cylindrical diode laser diffuser [86]. Subjects and observers were more satisfied with results after brachytherapy than PDT; however, subjects had a positive general impression after PDT. Adverse effects of burning were present for all subjects during interstitial illumination treatments necessitating intravenous opioids. Topical PDT sessions were better tolerated. Bu et al. preformed a prospective trial comparing surgery and superficial X-ray radiation therapy vs. surgery, superficial X-ray radiation therapy, and PDT in the split scar study in 10 subjects [85]. Both treatments noted significant symptom reduction. Only 1 keloid was painful at baseline which was relieved in both treatment groups by 6-month follow-up but reappeared in the treatment of postoperative radiation alone at 20-month follow-up. One of the ten subjects experienced keloid recurrence at 20 months on both sides of the scar. Adverse effects of mild pain were noted with PDT as well as one blister developing after PDT. Mild hyperpigmentation was observed in 6 subjects at 6-month follow-up of both treatment groups with gradual relief by the 20-month follow-up. These studies highlighted that although PDT carries the adverse effect of pain, it can potentially be a beneficial adjunct therapy.
Radiotherapy
Surgical excision of keloids is a potential treatment for mature keloids after failure of first-line therapies. However, as a monotherapy, it is associated with a recurrence rate of up to 100% [87]. To reduce the risk of recurrence, combination treatment modalities have been used. Surgical excision followed by radiation therapy has been showed to be highly effective at reducing recurrence (Table 6). Reduction in fibroblast proliferation and suppression of collagen synthesis by downregulation of TGF-beta and histamine release from mast cells is thought to be the underlying mechanism of action. Typical side effects include dyschromia and telangiectasia.

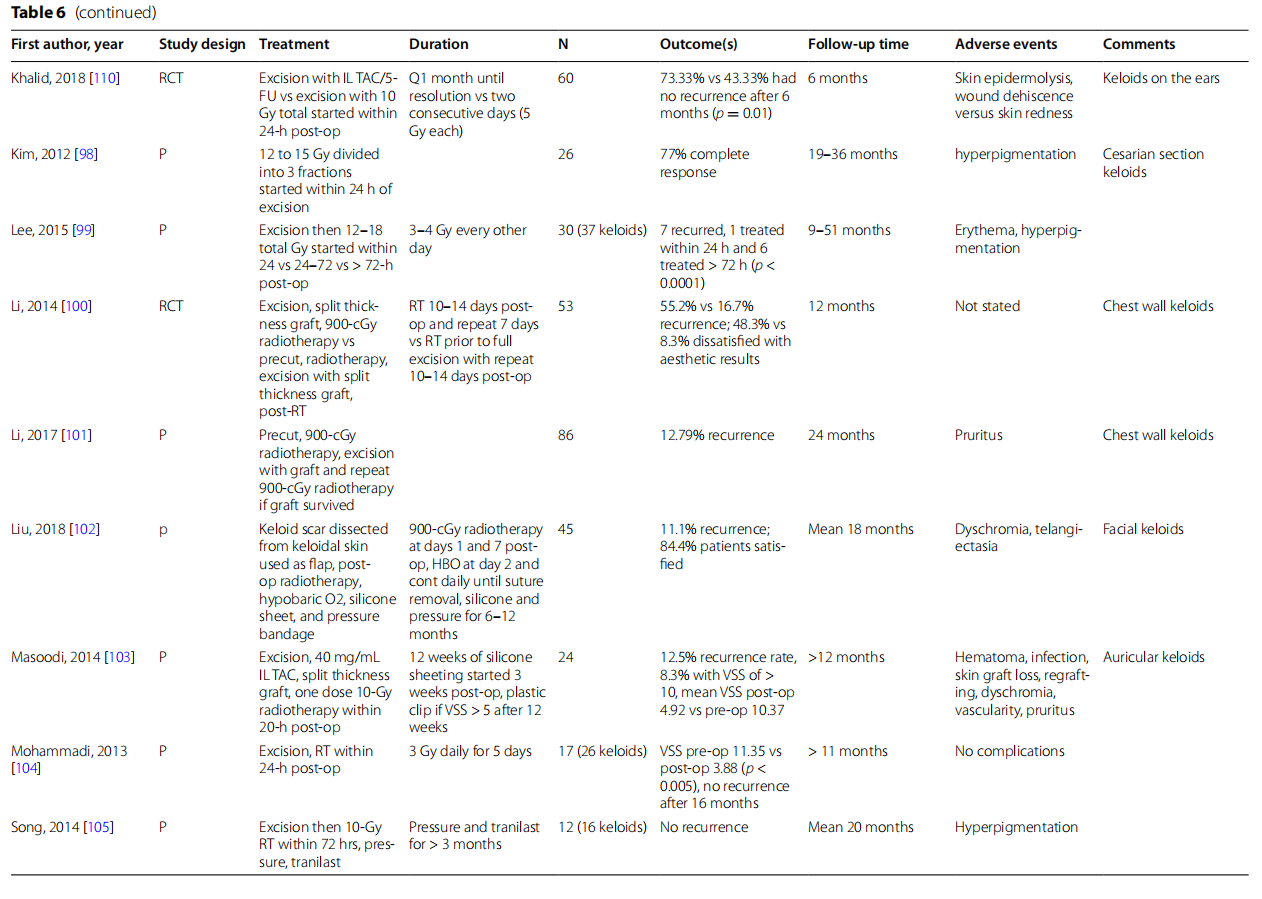
Direct comparisons of methods of keloid treatment are lacking. Aluko-Olokun et al. showed that IL TAC was superior to excision + RT in fattening facial keloids [88]. Similarly, Khalid et al. showed keloids treated with excision followed by IL TAC and 5-FU recurred in 8 of 30 subjects compared to 17 of 30 keloids treated with excision + RT at 6 months [110]. In contrast, Emad et al. found lower treatment failure and higher patient satisfaction with excision + RT than IL TAC and cryotherapy [90].
The majority of studies of excision + RT show administration of radiation within 24 h. Lee et al. compared timing of RT after excision. Of 37 keloids treated, 7 recurred with 1 being treated within 24 h and the other 6 treated after 72 h [99]. Tere have been a range of radiation doses and schedules investigated in the treatment of keloidal scars with no clear consensus on optimal dose and schedule. Recent evidence examining outcomes of keloids treated with excision and radiation therapy has recurrence rates ranging from no recurrence of the 26 and 16 treated keloids [104, 105] to 56.6% recurrence in 30 treated keloids [110]. Jiang et al. (2015 and 2018) showed low recurrence rates of 2 of 32 treated keloids (6%) and 3 of 37 keloids (8.1% )[95, 96], and Dunst et al. (2013) showed no recurrence with excision followed by 18 Gy of RT in 3 fractions over 36 h [89]. With the same total dose of radiation, Jones et al. showed a recurrence rate of 19% with RT divided over 4 days [97]. In another more extended schedule of radiation, Mohammadi et al. showed no recurrence over a minimum follow-up of 11 months for keloids treated with excision followed by 3 Gy of radiation daily for 5 days [104]. Vila Capel et al. demonstrated a higher 24% recurrence for excision followed by 15 Gy of radiation over 5 fractions given over 1 week using an electron beam with a novel aluminum spoiler [108].
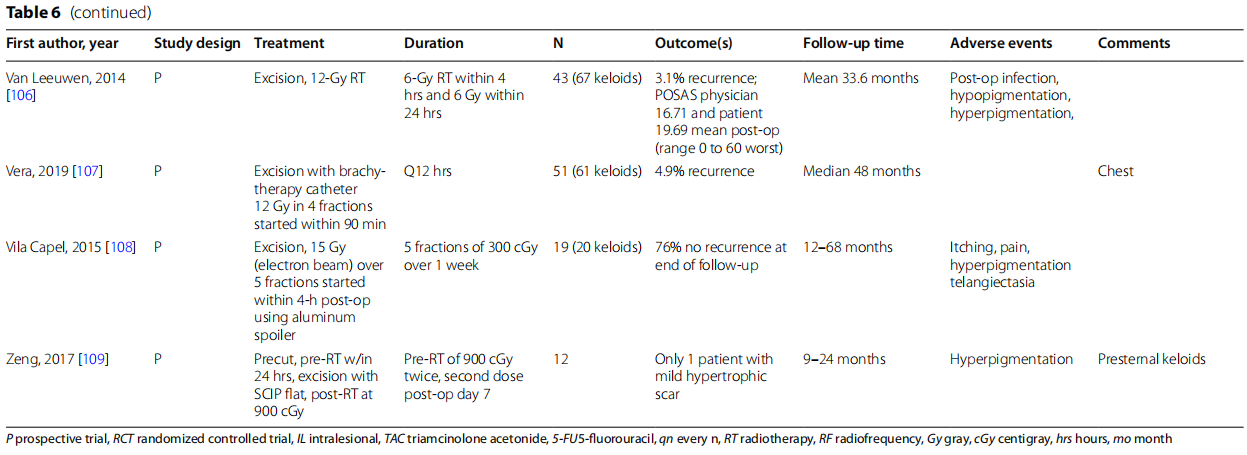
Van Leeuwen et al. found a recurrence rate of 3.1% with excision followed by 12 Gy of RT in two fractions within 24 h [106]. In contrast, 12–15 Gy of radiation divided into three fractions started within 24 h of excision for repeat C-section keloids showed a recurrence rate of 23% (Kim 2012) [98]. A single 13-Gy dose of brachytherapy within 2 h of excision from an implanted catheter also showed a similar rate of recurrence of 24% (Hafkamp 2017) [94]. Vera et al. showed a recurrence rate of 4.9% with excision followed by 12 Gy of brachytherapy in 4 fractions every 12 h (Vera, 2019) [105]. Song et al. also investigated a single radiation dose, showing no recurrence with excision followed by one dose of 10 Gy of radiation within 72 h and continued pressure therapy and oral tranilast (no dose specified, approved in Japan and South Korea) for greater than 3 months [105]. Combination of therapies showed a recurrence rate within the range seen for either excision or RT. Using a combination of excision, intraoperative intralesional triamcinolone, one dose of 10 Gy of radiation within 20 h of excision, and 12 weeks of silicone sheeting with pressure therapy if VSS was > 5 was shown to have a recurrence rate of 12.5% for auricular keloids (Masoodi 2014) [103].
Examining specifically chest wall keloids, studies have focused on precut and pre- and post-RT methods. Zeng et al. showed only one subject with mild hypertrophic scaring after a protocol of precutting for excision, two doses of pre-radiation, excision with fap repair, and postop RT [109]. Li et al. compared a similar precut method to more conventional excision + radiation for treatment of chest wall keloids [100]. Te pre-cut, pre-RT method was superior with a 16.7% recurrence rate compared to 55.2% with only post-excision radiation. In a larger study of this technique, Li et al. demonstrated a recurrence rate of 12.79% over 24 months of follow-up using the precut, pre-radiation method [101].
Liu et al. demonstrated a novel surgical technique of dissecting the keloid tissue from the overlying skin for use as a fap during repair [102]. Excision was followed by RT at days 1 and 7 post-op and hyperbaric oxygen at day 2. Continued silicone and pressure bandaging was used for 6–12 months. Over 18 months of follow-up, the recurrence rate was 11.1%.
Radiation as a monotherapy has also been investigated in the form of personalized patches containing either rhenium-188 or phosphorus-32. Subjects have generally shown fattening of their treated keloids with 59–77% showing > 50% fattening, with the highest percentages in those treated with a P-32 patch [91–93]. The side effects of treatment were radiation dermatitis, which was no different between the P-32 and Re-188 patches.
Silicone and pressure
Alteration of mechanical forces such as application of pressure or reduction of wound tension has been a long-standing treatment for keloids (Table 7). There has been sparse research examining the use of pressure as a monotherapy for keloids. One such study was a prospective noninvasive intervention study examining the daily application of traditionally worn tight clothing for 2 years conducted by Aluko-Olokun et al. [111] A mean volume reduction of 66.8% was seen in keloids with pedunculated lesions and 100% in keloids in sessile lesions. This study highlights the possible effectiveness of tight clothing as a noninvasive therapy for keloids, especially those with sessile morphology.
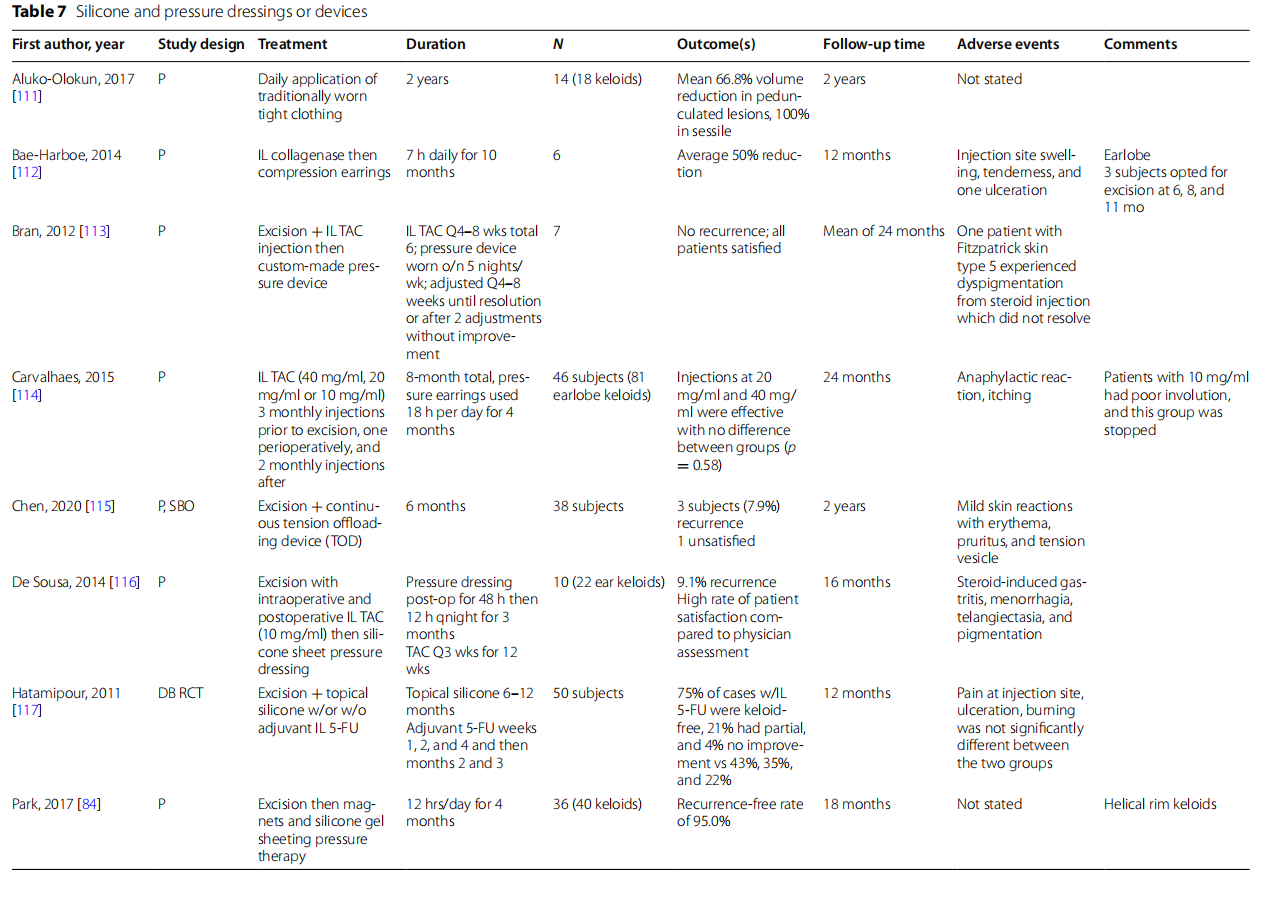
 Wound tension has been implicated in the pathogenesis of keloid formation. Chen et al. examined the use of a tension offloading device (TOD) applied for 6 months immediately after surgical excision [115]. After 2 years of follow-up, 35 of 38 subjects achieved healing with no recurrence. The use of the TOD requires high patient compliance. According to the authors, the 3 subjects that experienced recurrence in the study were noncompliant with recommended guidelines for TOD use. A prospective observational study by Tanaydin et al. followed 28 subjects that underwent surgical excision followed by application of a custom molded adjustable pressure clip to be worn 12 to 16 h per day for an average of 12–15 months [118]. In the group that reported nonrecurrence (71%), subjects were more compliant with therapy compared to the recurrence group. Another method of applying adjustable pressure is through magnets as studied by Park et al. where the outcomes of 40 subjects undergoing surgical excision of pure helical rim keloids followed by silicone gel sheets sandwiched between magnets for 12 h a day for 4 months were recorded [82]. At 18-month follow-up, there was a recurrence-free rate of 95% alongside a significant reduction in pain, itch, stiffness, thickness relief, and pliability on POSAS; no adverse events were reported.
Wound tension has been implicated in the pathogenesis of keloid formation. Chen et al. examined the use of a tension offloading device (TOD) applied for 6 months immediately after surgical excision [115]. After 2 years of follow-up, 35 of 38 subjects achieved healing with no recurrence. The use of the TOD requires high patient compliance. According to the authors, the 3 subjects that experienced recurrence in the study were noncompliant with recommended guidelines for TOD use. A prospective observational study by Tanaydin et al. followed 28 subjects that underwent surgical excision followed by application of a custom molded adjustable pressure clip to be worn 12 to 16 h per day for an average of 12–15 months [118]. In the group that reported nonrecurrence (71%), subjects were more compliant with therapy compared to the recurrence group. Another method of applying adjustable pressure is through magnets as studied by Park et al. where the outcomes of 40 subjects undergoing surgical excision of pure helical rim keloids followed by silicone gel sheets sandwiched between magnets for 12 h a day for 4 months were recorded [82]. At 18-month follow-up, there was a recurrence-free rate of 95% alongside a significant reduction in pain, itch, stiffness, thickness relief, and pliability on POSAS; no adverse events were reported.
The use of adjuvant therapy following surgical excision and application of pressure dressings has also been studied. Hatamipour et al. preformed a double-blinded randomized control trial comparing surgical excision with topical silicone vs adjuvant treatment with 5-FU [117]. At 1-year follow-up, 75% of subjects receiving all three therapies were keloid-free. Similarly, there have been studies examining adjuvant TAC injection with pressure therapy. De Sousa et al. performed a study examining surgical excision with intraoperative and postoperative TAC injection every 3 weeks for 12 weeks as well as silicone pressure dressing applied postoperatively for 48 h [116]. Keloid recurrence of 9.1% was seen at the end of follow-up at 16 months. Carvalhaes et al. also examined the use of intralesional TAC given before excision, perioperatively, and postoperatively [114]. Pressure earrings were used following excision in all groups. IL TAC at 20 mg/ml and 40 mg/ml were effective with no difference between groups. In a study by Bran et al., 7 subjects that underwent surgical excision of auricular keloids with corticosteroid injection followed by application of a custommade pressure device had complete resolution with no recurrence at 2-years follow-up [113]. Bae-Harboe et al. examined injection of collagenase Clostridium histolyticum to earlobe keloids followed by use of compression earrings [112]. An average of 50% reduction was seen in all keloids.
Other treatments
Recent prospective studies have focused on novel treatment methods (Table 8). Extracorporeal shockwave therapy (ESWT) as a monotherapy for keloids showed a reduction in volume, height, and appearance that was not significantly different compared to intralesional triamcinolone [119]. When ESWT was combined with IL TAC, Kim et al. noted a significant improvement in VSS compared to IL TAC alone, with no significant difference in side effects [120]. Further long-term studies of the effect of ESWT would be interesting as an additional treatment modality prior to excision. Application of a drug-free solid microneedle array found that after 4 weeks of treatment, there was a transient decrease in volume without a difference in VSS compared to an untreated control [121]. The treatment modality was well tolerated, but given that the volume improvement was lost, it is unclear what, if any, therapy duration would be needed for a durable clinical response. Finally, a custom radiotherapy patch led to durable symptomatic improvement and reduction in size in elevation [122]. Further studies will be needed to show how well these patches perform compared to standards of care such as IL TAC. Radiofrequency, most often used in cosmetic procedures such as micro-needling as well as ablative procedures for malignancy, was combined with IL TAC for the treatment of keloids. Weshay et al. treated 21 subjects with 3 to 4 sessions of radiofrequency and then IL TAC, and of the 18 subjects who completed the study, there was a 95.4% reduction in mean volume [123].
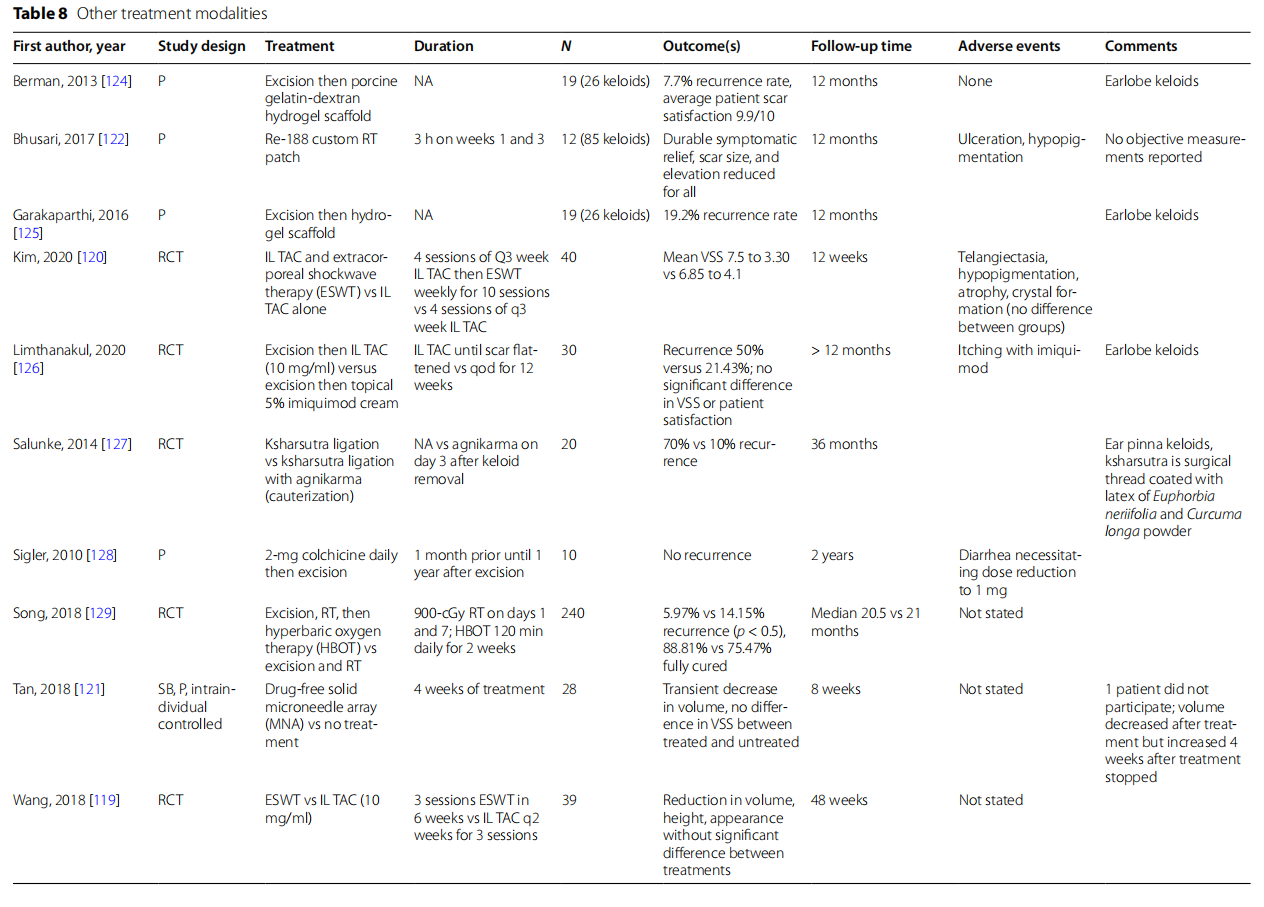

Many new treatment modalities were investigated as adjunctive therapy with excision. Oral colchicine taken 1 month prior to excision until 1 year after impressively found no recurrence during the follow-up period, though only 10 subjects were treated (Sigler 2010) [128]. Excision with IL-TAC until scar fattening was compared to postexcision 5% topical imiquimod every other night for 12 weeks, showing a reduction in recurrence from 50 to 21.43% [126]. Berman et al. found a very promising recurrence rate of 7.7% for keloids treated with excision and then placement of a porcine hydrogel scaffold [124]. Similarly, Garakaparthi et al. showed a 19.2% recurrence rate with excision and then administration of a hydrogel scaffold for treatment of ear lobe keloids [125]. To improve upon the low recurrence rates of excision followed by RT, Song et al. investigated the addition of hyperbaric oxygen therapy daily for 2 weeks in addition to excision and RT and found it reduced the recurrence rate to 5.9% compared to 14.15% with excision and RT alone [129]. Lastly, Salunke et al. showed that a ligation with cauterization method reduced the recurrence rate from 70% with ligation alone to 10% [127].
Discussion and recommendations
Pressure and silicone-based therapies have long-standing data behind their efficacy and safety when used both as prevention after surgery and treatment of established keloids, as has been noted by multiple recent consensus guidelines [130]. Recent evidence contributes similar results to the collective literature, showing silicone dressings decreased recurrence while being both safe and well tolerated. Only one recent study examined pressure therapy without excision. Bae-Harboe showed a 50% improvement with pressure earring applied after intralesional collagenase administration. Flatter lesions would likely respond better in combination with corticosteroid impregnated tape and silicone dressings [131]; however, no recent studies have compared these modalities. Overall, these studies highlighted that the key to effectiveness of compression therapy may lie in compliance as well as providing adequate levels of pressure. Limitations to pressure therapy include conspicuous nature of devices, keloid morphology, and patient comfort. Pressure therapy may provide some effect for those looking for conservative treatment for keloids, but effectiveness is increased with combination therapy and with adjustable pressure devices worn for at least periods of 12 h. As ways to manipulate mechanical pressure to treat keloids are explored, the reduction of tension utilizing special tension offloading devices shows promise.
For established keloids, intralesional corticosteroids are the first-line treatment with or without additional therapeutics topically or intralesionally, as is recommended by many consensus guidelines [131–134]. Recent studies have focused on how best to administer IL TAC. Optimal interval timing between injections was suggested to be 2 weeks, though standard of care is typically 4–6 weeks, so further studies confirming this will be needed to change clinical practice. As clinically suspected, sessile lesions were found to respond better to IL TAC compared to pedunculated keloids. The role of IL TAC as an adjuvant to surgical excision continues to have conflicting results in the literature, and some studies lack a control group, making it difficult to recommend compared to methods such as excision with adjunctive RT, which has consistently low recurrence rates.
Other intralesional injections including botulinun toxin A (BTA), bleomycin, mitomycin C, PRP, and collagenase have been recently investigated. The success of treatment with verapamil is mixed and treatment both as an intralesional therapy or as an adjuvant to cryotherapy or excision; verapamil has not consistently outperformed IL TAC. However, verapamil is well-tolerated, so likely lower risk of adverse events. 5-FU has been extensively studied, and recent literature has confirmed synergy in treatment with IL TAC, outperforming either treatment alone in multiple comparative studies, though does have an increased risk of ulceration. 5-FU tattooing has shown promising results, outperforming IL-TAC in a randomized double-blinded study. In recent studies, bleomycin did not outperform IL TAC and had an increased risk of bulla and ulceration. Interestingly, BTA outperformed 5-FU alone and was found to have no difference compared to IL TAC in a double-blinded study, with a low risk of hypopigmentation. Surgical excision with adjuvant cryotherapy and PRP showed a recurrence rate of 16.21%, though since the study had no control group, further study is needed to recommend PRP.
Intralesional cryotherapy is recommended for smaller lesions [131]. Recent comparative studies have shown that intralesional cryotherapy was less effective than excision + IL TAC or excision + RT for resistant keloids, leading to early termination of the trial. Intralesional cryotherapy was shown to have better clinical improvement in two recent studies. Intralesional cryotherapy is a better option for keloids with greater thickness and are not optimal candidates for excision.
Light-based treatment, most commonly PDL or ablative laser therapy, has been recommended as a second-line therapy prior to excision [132]. Fractional CO2 showed no difference in improvement compared to IL verapamil or TAC, and efficacy of CO2 laser with IL TAC compared to cryotherapy with IL TAC was not significantly different. Given the cost and access barriers, laser is likely best in combination with IL or topical CS therapy for the best clinical outcomes shown by multiple recent studies showing improvement with laser treatment followed by IL TAC and/or 5-FU [79–81]. Laser-assisted delivery of corticosteroids and combination of different lasers for treatment of keloids are emerging treatments. Recent studies have shown comparable or slightly improved results with Er:YAG or CO2 followed by topical corticosteroid and occlusion as compared with IL TAC alone or IL TACwith laser. PDT is another emerging application in the field of keloid treatment, though excision followed by PDT has not been found to be more effective than RT.
Excision followed by radiation therapy has been shown to consistently reduce the risk of recurrence. Comparison showed a higher response rate and lower adverse effects compared to cryotherapy with IL TAC. Brachytherapy and externally applied radiation have both shown success with no head-to-head trials. Most successful RT protocols deliver 12–18 Gy over 3–5 days with the optimal timing of radiation beginning within 24 h of excision. For pre-ternal keloids, a specialized method of pre-cut for excision followed by pre-radiation and post-radiation after excision showed a significantly reduced recurrence rate compared to excision with post-radiation only. Radiation therapy alone has shown symptomatic improvement and some success in fattening lesions, but recent studies have not compared it to other first-line therapies such as IL TAC.
Recent investigations of novel treatments have had some promising results. Application of a hydrogel scaffold after excision had low recurrence rates, though have not yet been compared in randomized comparative trials. Both drug-loaded and drug-free microneedle arrays have been tried as a less invasive and painful option, but the clinical improvement has not been shown to be durable as a monotherapy. ESWT with and without IL TAC has been shown to have similar results to IL TAC, which shows promise and warrants further investigation. Topical imiquimod after excision was shown to have reduced recurrence compared to excision with IL TAC, which is a good option for accessible lesions such as ear keloids. Colchicine as an oral therapy started 1 month prior to excision showed no recurrence and was well tolerated, which is a promising systemic therapy option.
Limitations
Although potential treatments for keloids range from topical and injectable therapeutics to surgical interventions and light therapies, there is no one consistent method of treatment that can guarantee response to therapy and prevent recurrence. Evidence for therapies lack consistent controls, and outcomes are heterogeneous, making it difficult to compare outcomes across studies. Heterogeneity of subject characteristics such as family history, keloid location, skin tension, size, and number, as well as gender and Fitzpatrick skin type, could all play a role in keloid response. Tere are many novel and effective treatments not included in this review, as non-English language studies, databases from other fields (such as nursing), case studies, case series, and retrospective studies and reviews were excluded from this review of - the past decade of investigation. The field of keloid treatment would benefit from consistent, validated outcomes. - There are multiple standardized tools for the assessment of keloids including the Patient and Observer Scare Assessment Scale, the Vancouver Scar Scale, and the JSW Scar Scale, and objective measurements of dimensions, color, pliability, and perfusion can be compared [135]. - Both subject-controlled and split scar studies are success full controls, and randomization with at least evaluator - blinding will improve the quality of evidence. Patient sat isfaction and quality of life can also be assessed with the Dermatology Life Quality Index.
Conclusions
Keloids are a pathologic scarring response to dermal injury that progress to involve normal tissue outside the original injury and have a significant impact on quality of life. With multiple treatment modalities available, first- line therapy is silicone gel or sheeting with corticoster-oid injections for more tumoral lesions or tape for fatter keloids. Providers can consider adjuvant intralesional 5-FU, bleomycin, or verapamil depending on patient preference and side effect profile. Laser therapy can be considered in combination with intralesional injection of corticosteroids or topical steroids with occlusion. For keloids that inadequately respond, excision with RT of 16–20 Gy over a maximum of 5 days started within 24 h can be considered. Additional treatment with silicone - sheeting and pressure therapy is reasonable with possible oral colchicine to prevent recurrence. As the field- continues to progress in the understanding of keloid etiology, the promise of new therapeutic targets and more specialized treatment regimens emerges.
Supplementary Information
The online version contains supplementary material available at https://doi. org/10.1186/s13643-023-02192-7.
Additional file 1. RoB 2 by each study.
Additional file 2. ROBINS-I by each study.
Authors’ contributions
The authors read and approved the final manuscript.
Funding
The authors have no funding sources to disclose.
Declarations
Competing interests
The authors declare that they have no competing interests.
Received: 29 May 2022 Accepted: 15 February 2023
References
1. Robles DT, Berg D. Abnormal wound healing: keloids. Clin Dermatol. 2007;25( 1):26- -32.
2. Bolognia 儿, Schaffer JV, Cerroni L, Callen JP. Dermatology. 4th ed: Elsevier; 2018.
3. English RS, Shenefelt PD. Keloids and hypertrophic scars. Dermatol Surg.1999;25:8.
4. Nemeth AJ. Keloids and hypertrophic scars. J Dermatol Surg Oncol. 1993;1 9(8):738- -46.
5. Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int J Mol Sci. 2017;1 8(3):606.
6. Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 201 7;43(Suppl 1):S3-s18.
7. Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;1 17(1):286-300.
8. Lee SS, Yosipovitch G, Chan YH, Goh CL. Pruritus, pain, and small nerve fiber function in keloids: a controlled study. J Am Acad Derma-tol.2004;51(6):1002-6.
9. Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297(10):433-8.
10. Balci DD, Inandi T, Dogramaci CA, Celik E. DLQI scores in patients with keloids and hypertrophic scars: a prospective case control study. Journal der Deutschen Dermatologischen Gesellschaft. 2009;7(8):688- -91.
11. Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics: part I. Strategies to reduce scar formation after dermatologic procedures. J Am
Acad Dermatol. 201 2;66(1):13-24.
12. BaoY, XuS, Pan Z, DengJ,LiX, Pan F, et al. Comparative efficacy and safety of common therapies in keloids and hypertrophic scars: a systematic review
and meta-analysis. Aesthet Plast Surg. 2020;44(1):207-18.
13. Betarbet U, Blalock TW. Keloids: a review of etiology, prevention, and treatment. J Clin Aesthetic Dermatol. 2020;1 3(2):33- -43.
14. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898.
15. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-l: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 20 16;355:14919.
16. McGuinness LA, Higgins JPT. Risk- of-bias VISualization (robvis):an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55-61.
17. Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol.
2018;11:387- -96.
18. Acosta S, Ureta E, Yanez R, Oliva N, Searle S, Guerra C. Effectiveness of intralesional triamcinolone in the treatment of keloids in children. Pediatr Dermatol. 2016;33(1):75- -9.
19. Aluko-Olokun B, Olaitan AA, Ladeinde AL Sessile and pedunculated facial keloid scar: a comparison of response to intralesional triamci-nolone injection. Eur J Plast Surg. 2014;37(5):255- -8.
20. Aluko-Olokun B, Olaitan AA, Aluko-Olokun OA. Injection complications and change in keloid height following intralesional injection of lesions: a novel injection system compared with the traditional method. Eur J
Plast Surg.201 5;38(5):397- -404.
21. Aluko-Olokun B, Olaitan AA, L adeinde AL, Aluko-Olokun OA, Alade MO, lbukun-Obaro O, et al. Determination of the optimal frequency of injection of triamcinolone: monitoring change in volume of keloid
lesions fallowing injection of 40 mg of triamcinolone. Eur J Plast Surg. 2016;39(2):119-24.
22. Aluko-Olokun B, Olaitan AA,Morgan RE, Adediran OM. Preventionof earlobe keloid recurrence after excision: assessment of the value of presurgical injection of triamcinolone. J Craniofac Surg. 2018;29(7):e673-e5.
23. Bashir MM, Ahmad H, Yousaf N, Khan FA. Comparison of single intra operative versus an intra operative and two post operative injections of the triamcinolone after wedge excision of keloids of helix. J Pak Med
Assoc.2015;65(7):737-41.
24. Brown NA, Ortega FR. The role of full-thickness skin grafting and steroid injection in the treatment of auricular keloids. Ann Plast Surg. 2010;64(5):637–8.
25. Chua SC, Gidaszewski B, Khajehei M. Efcacy of surgical excision and sub-dermal injection of triamcinolone acetonide for treatment of keloid scars after caesarean section: a single blind randomised controlled trial protocol. Trials. 2019;20(1):363.
26. dos Santos JM, de Souza C, de Vasconcelos AC, Nunes TA. Effect of triamcinolone in keloids morphological changes and cell apoptosis. Rev Col Bras Cir. 2015;42(3):171–4.
27. Farkhad RI, Noreen J, Ashraf S. Keloid still a challenge? Pak J Med Health Sci. 2012;6(4):921–3.
28. Huu ND, Huu SN, Thi XL, Van TN, Minh PPT, Minh TT, et al. Successful treatment of intralesional triamcilonon acetonide injection in keloid patients. Open Access Maced J Med Sci. 2019;7(2):275–8.
29. Kaushal V, Kumar S, Brar BK, Singh A. Comparative evaluation of therapeutic efficacy and safety of intralesional triamcinolone acetonide injection vs intralesional radiofrequency with intralesional triamcinolone acetonide in treatment of keloids. Dermatol Ther. 2020;33(6):e13919.
30. Nor NM, Ismail R, Jamil A, Shah SA, Imran FH. A randomized, singleblind trial of clobetasol propionate 0.05% cream under silicone dressing occlusion versus intra-lesional triamcinolone for treatment of keloid.
Clin Drug Investig. 2017;37(3):295–301.
31. Schwaiger H, Reinholz M, Poetschke J, Ruzicka T, Gauglitz G. Evaluating the therapeutic success of keloids treated with cryotherapy and intralesional corticosteroids using noninvasive objective measures. Dermatol Surg. 2018;44(5):635–44.
32. Tan CWX, Tan WD, Srivastava R, Yow AP, Wong DWK, They HL. Dissolving triamcinolone-embedded microneedles for the treatment of keloids: a single-blinded intra-individual controlled clinical trial. Dermatol Ther (Heidelb). 2019;9(3):601–11.
33. They HL, Tan CW. Novel microneedle treatment for keloids: effects on lesional volume, pain and itch. Acta Derm Venereol. 2017;97(8):1053.
34. They HL, Tan CW, Lim CY, Tan VW. Corticosteroid-embedded dissolving microneedles for dermatological treatment. Allergy: European. J Allergy Clin Immunol. 2018;73:731–2.
35. Tripoli M, Cordova A, Melloni C, Zabbia G, Maggì F, Moschella F. The use of triamcinolone combined with surgery in major ear keloid treatment: a personal two stages approach. Eur J Plast Surg. 2015;38(3):205–10.
36. Cai L, Hu M, Lin L, Zheng T, Liu J, Li Z. Evaluation of the effcacy of triamcinolone acetonide in the treatment of keloids by high-frequency ultrasound. Skin Res Technol. 2020;26(4):489–93.
37. Dalkowski A, Fimmel S, Beutler C, Zouboulis CC. Cryotherapy modifes synthetic activity and differentiation of keloidal fibroblasts in vitro. Exp Dermatol. 2003;12(5):673–81.
38. Har-Shai Y, Sabo E, Rohde E, Hyams M, Assaf C, Zouboulis CC. Intralesional cryosurgery enhances the involution of recalcitrant auricular keloids: a new clinical approach supported by experimental studies. Wound Repair Regen. 2006;14(1):18–27.
39. Barara M, Mendiratta V, Chander R. Cryotherapy in treatment of keloids: evaluation of factors affecting treatment outcome. J Cutan Aesthet Surg. 2012;5(3):185–9.
40. Jannati P, Aref S, Amin Jannati A, Jannati F, Moravvej H. Comparison of therapeutic response of keloids to cryotherapy plus intralesional triamcinolone acetonide or verapamil hydrochloride. J Ski Stem Cell. 2015;2(1):e29284.
41. Fraccalvieri M, Bogetti P, Salomone M, Di Santo C, Ruka E, Bruschi S. Cryotreatment of keloids: a single Italian institution experience. Eur J Plast Surg. 2016;39(3):201–6.
42. Careta MF, Fortes AC, Messina MC, Maruta CW. Combined treatment of earlobe keloids with shaving, cryosurgery, and intralesional steroid injection: a 1-year follow-up. Dermatol Surg. 2013;39(5):734–8.
43. Azzam EZ, Omar SS. Treatment of auricular keloids by triple combination therapy: surgical excision, platelet-rich plasma, and cryosurgery. J Cosmet Dermatol. 2018;17(3):502–10.
44. Abdel-Meguid AM, Weshahy AH, Sayed DS, Refaiy AE, Awad SM. Intralesional vs. contact cryosurgery in treatment of keloids: a clinical and immunohistochemical study. Int J Dermatol. 2015;54(4):468–75.
45. Bijlard E, Timman R, Verduijn GM, Niessen FB, van Neck JW, Busschbach JJ, et al. Intralesional cryotherapy versus excision and corticosteroids or brachytherapy for keloid treatment: study protocol for a randomised controlled trial. Trials. 2013;14:439.
46. Bijlard E, Timman R, Verduijn GM, Niessen FB, Hovius SER, Mureau MAM. Intralesional cryotherapy versus excision with corticosteroid injections or brachytherapy for keloid treatment: Randomised controlled trials. J Plast Reconstr Aesthet Surg. 2018;71(6):847–56.
47. Mourad B, Elfar N, Elsheikh S. Spray versus intralesional cryotherapy for keloids. J Dermatolog Treat. 2016;27(3):264–9.
48. Patni G, Velurethu R, Shamanur M, Viswanath B. Intralesional cryotherapy for enhancing the involution of keloids: a clinical study. Br J Dermatol. 2017;177:106.
49. van Leeuwen MC, Bulstra AE, van Leeuwen PA, Niessen FB. A new argon gas-based device for the treatment of keloid scars with the use of intralesional cryotherapy. J Plast Reconstr Aesthet Surg. 2014;67(12):1703–10.
50. Weshahy AH. Intralesional cryosurgery. A new technique using cryoneedles. J Dermatol Surg Oncol. 1993;19(2):123–6.
51. Abou-Taleb DAE, Badary DM. Intralesional verapamil in the treatment of keloids: a clinical, histopathological, and immunohistochemical study. J Cosmet Dermatol. 2020;20(1):267–73.
52. Aggarwal A, Ravikumar BC, Vinay KN, Raghukumar S, Yashovardhana DP. A comparative study of various modalities in the treatment of keloids. Int J Dermatol. 2018;57(10):1192–200.
53. Ali H, Siddique M, Pervez M, Kumar S, Sami W. Comparison of 5 fluorouracil and triamcinolone acetonide intralesional injection in the management of keloid. Rawal Med J. 2020;45(3):549–53.
54. Danielsen PL, Rea SM, Wood FM, Fear MW, Viola HM, Hool LC, et al. Verapamil is less effective than triamcinolone for prevention of keloid scar recurrence after excision in a randomized controlled trial. Acta Derm Venereol. 2016;96(6):774–8.
55. El-Kamel MF, Selim MK, Alghobary MF. Keloidectomy with core fillet fap and intralesional verapamil injection for recurrent earlobe keloids. Indian J Dermatol Venereol Leprol. 2016;82(6):659–65.
56. Gamil HD, Khattab FM, El Fawal MM, Eldeeb SE. Comparison of intralesional triamcinolone acetonide, botulinum toxin type a, and their combination for the treatment of keloid lesions. J Dermatolog Treat. 2020;31(5):535–44.
57. Hewedy ES, Sabaa BEI, Mohamed WS, Hegab DS. Combined intralesional triamcinolone acetonide and platelet rich plasma versus intralesional triamcinolone acetonide alone in treatment of keloids. J Dermatolog Treat. 2022;33(1):150–6.
58. Ismail SA, Mohammed NHK, Sotohy M, Abou-Taleb DAE. Botulinum toxin type A versus 5-Fluorouracil in treatment of keloid. Arch Dermatol Res. 2021;313(7):549–56.
59. Khan HA, Sahibzada MN, Paracha MM. Comparison of the efficacy of intralesional bleomycin versus intralesional triamcinolone acetonide in the treatment of keloids. Dermatol Ther. 2019;32(5):e13036.
60. Khare N, Patil SB. A novel approach for management of ear keloids: results of excision combined with 5-fuorouracil injection. J Plast Reconstr Aesthet Surg. 2012;65(11):e315–7.
61. Khattab FM, Nasr M, Khashaba SA, Bessar H. Combination of pulsed dye laser and verapamil in comparison with verapamil alone in the treatment of keloid. J Dermatolog Treat. 2020;31(2):186–90.
62. Pruksapong C, Yingtaweesittikul S, Burusapat C. Efficacy of botulinum toxin a in preventing recurrence keloids: double blinded randomized controlled trial study: intraindividual subject. J Med Assoc Thail.2017;100(3):280–6.
63. Rasaii S, Sohrabian N, Gianfaldoni S, Hadibarhaghtalab M, Pazyar N, Bakhshaeekia A, et al. Intralesional triamcinolone alone or in combination with botulinium toxin a is ineffective for the treatment of formed keloid scar: a double blind controlled pilot study. Dermatol Ther. 2019;32(2):e12781.
64. Reinholz M, Guertler A, Schwaiger H, Poetschke J, Gauglitz GG. Treatment of keloids using 5-fuorouracil in combination with crystalline triamcinolone acetonide suspension: evaluating therapeutic effects by using non-invasive objective measures. J Eur Acad Dermatol Venereol. 2020;34(10):2436–44.
65. Sadeghinia A, Sadeghinia S. Comparison of the efficacy of intralesional triamcinolone acetonide and 5-fuorouracil tattooing for the treatment of keloids. Dermatol Surg. 2012;38(1):104–9.
66. Sagheer A, Shehzad A, Hussain I. Comparison of efficacy of intralesional 5-fuorouracil alone versus intralesional triamcinolone acetonide with 5-fuorouracil in small keloids. J Pak Assoc Dermatol. 2016;26(4):361–5.
67. Saha AK, Mukhopadhyay M. A comparative clinical study on role of 5-furouracil versus triamcinolone in the treatment of keloids. Indian J Surg. 2012;74(4):326–9.
68. Saki N, Mokhtari R, Nozari F. Comparing the efficacy of intralesional triamcinolone acetonide with verapamil in treatment of keloids: a randomized controlled trial. Dermatol Pract Concept. 2019;9(1):4–9.
69. Saleem F, Rani Z, Bashir B, Altaf F, Khurshid K, Pal SS. Comparison of efficacy of intralesional 5-fuorouracil plus triamcinolone acetonide versus intralesional triamcinolone acetonide in the treatment of keloids. J Pak Assoc Dermatol. 2017;27(2):114–9.
70. Shaarawy E, Hegazy RA, Abdel Hay RM. Intralesional botulinum toxin type a equally effective and better tolerated than intralesional steroid in the treatment of keloids: a randomized controlled trial. J Cosmet Dermatol. 2015;14(2):161–6.
71. Srivastava S, Kumari H, Singh A. Comparison of fractional CO(2) laser, verapamil, and triamcinolone for the treatment of keloid. Adv Wound Care (New Rochelle). 2019;8(1):7–13.
72. Velurethu R, Viswanath B, Shamanur M, Patni G. Triple medicine combination injection, a new cocktail to combat keloids in the present era: a
prospective clinical study. Br J Dermatol. 2017;177:105–6.
73. Wilson AM. Eradication of keloids: surgical excision followed by a single injection of intralesional 5-fuorouracil and botulinum toxin. Can J Plast Surg. 2013;21(2):87–91.
74. Huu ND, Huu SN, Thi XL, Van TN, Minh PPT, Minh TT, et al. Successful treatment of intralesional bleomycin in keloids of vietnamese population. Open Access Maced J Med Sci. 2019;7(2):298–9.
75. Srivastava S, Patil AN, Prakash C, Kumari H. Comparison of intralesional triamcinolone acetonide, 5-fuorouracil, and their combination for the treatment of keloids. Adv Wound Care (New Rochelle). 2017;6(11):393–400.
76. Abd El-Dayem DH, Nada HA, Hanafy NS, Elsaie ML. Laser-assisted topical steroid application versus steroid injection for treating keloids: a split side study. J Cosmet Dermatol. 2020;20(1):138–42.
77. Annabathula A, Sekar CS, Srinivas CR. Fractional carbon dioxide, long pulse Nd:YAG and pulsed dye laser in the management of keloids. J Cutan Aesthet Surg. 2017;10(2):76–80.
78. Behera B, Kumari R, Thappa DM, Malathi M. Therapeutic efficacy of intralesional steroid with carbon dioxide laser versus with cryotherapy in treatment of keloids: a randomized controlled trial. Dermatol Surg.
2016;42(10):1188–98.
79. Chen XE, Liu J, Bin Jameel AA, Valeska M, Zhang JA, Xu Y, et al. Combined effects of long-pulsed neodymium-yttrium-aluminum-garnet laser, diprospan and 5-fuorouracil in the treatment of keloid scars. Exp Ther Med. 2017;13(6):3607–12.
80. Garg GA, Sao PP, Khopkar US. Effect of carbon dioxide laser ablation followed by intralesional steroids on keloids. J Cutan Aesthet Surg. 2011;4(1):2–6.
81. Kassab AN, El Kharbotly A. Management of ear lobule keloids using 980-nm diode laser. Eur Arch Otorhinolaryngol. 2012;269(2):419–23.
82. Park TH, Rah DK. Successful eradication of helical rim keloids with surgical excision followed by pressure therapy using a combination of magnets and silicone gel sheeting. Int Wound J. 2017;14(2):302–6.
83. Wang J, Wu J, Xu M, Gao Q, Chen B, Wang F, et al. Combination therapy of refractory keloid with ultrapulse fractional carbon dioxide (CO(2)) laser and topical triamcinolone in Asians-long-term prevention of keloid recurrence. Dermatol Ther. 2020;33(6):e14359.
84. Park JH, Chun JY, Lee JH. Laser-assisted topical corticosteroid delivery for the treatment of keloids. Lasers Med Sci. 2017;32(3):601–8.
85. Bu W, Fang F, Zhang M, Chen J. Combination of 5-ALA photodynamic therapy, surgery and superficial X-ray for the treatment of keloid. Photodermatol Photoimmunol Photomed. 2020;36(1):65–7.
86. Basdew H, Mehilal R, Al-Mamgani A, Van Rooij P, Bhawanie A, Sterenborg HJCM, et al. Adjunctive treatment of keloids: comparison of photodynamic therapy with brachytherapy. Eur J Plast Surg. 2013;36(5):289–94.
87. Niessen FB, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104(5):1435–58.
88. Aluko-Olokun B, Olaitan AA, Ladeinde AL, Oginni FO. The facial keloid: a comparison of treatment outcome between intralesional steroid injection and excision combined with radiotherapy. Eur J Plast Surg. 2014;37(7):361–6.
89. Dunst J, Jiang P, Niehof P, Bockelmann G, Druecke D, Siebert F. Adjuvant HDR-brachytherapy for treatment of recurrent keloids. Int J Radiat Oncol Biol Phys. 2013;87(2):S559–S60.
90. Emad M, Omidvari S, Dastgheib L, Mortazavi A, Ghaem H. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: a prospective clinical trial. Med Princ Pract. 2010;19(5):402–5.
91. Gupta P, Verma KK, Lochab SP, Kumar P, Malhotra A, Bandopadhyaya GP, et al. Treatment of keloids using re-188: a pilot study. Eur J Nucl Med Mol Imaging. 2012;39:S610–S1.
92. Gupta P, Malhotra A, Verma KK, Lochab SP, Gupta S, Kumar P, et al. Patch radiation therapy : breakthrough in keloid management. Eur J Nucl Med Mol Imaging. 2013;40:S410.
93. Gupta P, Verma KK, Kumar R, Kumar P, Malhotra A, Bandopadhyaya GP, et al. Re-188 patch radionuclide therapy for keloids: a 3 year follow up study. Eur J Nucl Med Mol Imaging. 2017;44(2):S804-S.
94. Hafkamp CJH, Lapid O, Davila Fajardo R, van de Kar AL, Koedooder C, Stalpers LJ, et al. Postoperative single-dose interstitial high-doserate brachytherapy in therapy-resistant keloids. Brachytherapy. 2017;16(2):415–20.
95. Jiang P, Baumann R, Dunst J, Geenen M, Siebert FA, Niehof P, et al. Perioperative interstitial high-dose-rate brachytherapy for the treatment of recurrent keloids: feasibility and early results. Int J Radiat Oncol Biol Phys. 2016;94(3):532–6.
96. Jiang P, Geenen M, Siebert FA, Bertolini J, Poppe B, Luetzen U, et al. Efficacy and the toxicity of the interstitial high-dose-rate brachytherapy in the management of recurrent keloids: 5-year outcomes. Brachytherapy. 2018;17(3):597–600.
97. Jones ME, Ganzer CA, Bennett D, Finizio A. Surgical excision of keloids followed by in-office superficial radiation therapy: prospective study examining clinical outcomes. Plast Reconstr Surg Glob Open.2019;7(5):e2212.
98. Kim J, Lee SH. Therapeutic results and safety of postoperative radiotherapy for keloid after repeated cesarean section in immediate postpartum period. Radiat Oncol J. 2012;30(2):49–52.
99. Lee SY, Park J. Postoperative electron beam radiotherapy for keloids: treatment outcome and factors associated with occurrence and recurrence. Ann Dermatol. 2015;27(1):53–8.
100. Li W, Wang Y, Wang X, Liu Z. A keloid edge precut, preradiotherapy method in large keloid skin graft treatment. Dermatol Surg. 2014;40(1):52–7.
101. Li W. Pre radiotherapy and its use in large keloid treatment. Med Phys. 2017;44(6):2823.
102. Liu S, Liang W, Song K, Wang Y. Keloid skin fap retention and resurfacing in facial keloid treatment. Aesthet Plast Surg. 2018;42(1):304–9.
103. Masoodi Z, Ahmad I, Khurram MF, Haq A. Excision, skin grafting, corticosteroids, adjuvant radiotherapy, pressure therapy, and emancipation: the ESCAPE model for successful taming of giant auricular keloids. Adv Skin Wound Care. 2014;27(9):404–12.
104. Mohammadi AA, Mohammadian Panah M, Pakyari MR, Tavakol R, Ahrary I, Seyed Jafari SM, et al. Surgical excision followed by low dose rate radiotherapy in the management of resistant keloids. World J Plast Surg. 2013;2(2):81–6.
105. Song C, Wu HG, Chang H, Kim IH, Ha SW. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J Radiat Res. 2014;55(5):912–6.
106. van Leeuwen MC, Stokmans SC, Bulstra AE, Meijer OW, van Leeuwen PA, Niessen FB. High-dose-rate brachytherapy for the treatment of recalcitrant keloids: a unique, effective treatment protocol. Plast Reconstr Surg. 2014;134(3):527–34.
107. Vera Barragam V, De Juan MM, Blanco Parajón S, Fernández García J,Juan Rijo G, Alonso García AI. Perioperative interstitial high dose rate brachytherapy for keloids scars. Radiother Oncol. 2019;133:S167.
108. Vila Capel A, Vilar Palop J, Pedro Olivé A, Sánchez-Reyes FA. Adjuvance in refractory keloids using electron beams with a spoiler: recent results. Rep Pract Oncol Radiother. 2015;20(1):43–9.
109. Zeng A, Song K, Zhang M, Men Q, Wang Y, Zhu L, et al. The "sandwich therapy": a microsurgical integrated approach for presternal keloid treatment. Ann Plast Surg. 2017;79(3):280–5.
110. Khalid FA, Farooq UK, Saleem M, Rabbani J, Amin M, Khan KU, et al.The efficacy of excision followed by intralesional 5-fuorouracil and triamcinolone acetonide versus excision followed by radiotherapy in the treatment of ear keloids: a randomized control trial. Burns. 2018;44(6):1489–95.
111. Aluko-Olokun B, Olaitan AA, Ladeinde AL, Oginni FO, Morgan RE,Aluko-Olokun OA, et al. Pressure therapy using tight-fitting garments: a comparison of response of sessile and pedunculated keloids in Africanwomen. Intern Med J. 2017;24(5):411–4.
112. Bae-Harboe YS, Harboe-Schmidt JE, Graber E, Gilchrest BA. Collagenase followed by compression for the treatment of earlobe keloids. Dermatol Surg. 2014;40(5):519–24.
113. Bran GM, Brom J, Hormann K, Stuck BA. Auricular keloids: combined therapy with a new pressure device. Arch Facial Plast Surg. 2012;14(1):20–6.
114. Carvalhaes SM, Petroianu A, Ferreira MA, de Barros VM, Lopes RV. Assesment of the treatment of earlobe keloids with triamcinolone injections, surgical resection, and local pressure. Rev Col Bras Cir. 2015;42(1):9–13.
115. Chen B, Ding J, Jin J, Song N, Liu Y. Continuous tension reduction to prevent keloid recurrence after surgical excision: preliminary experience in Asian patients. Dermatol Ther. 2020;33(4):e13553.
116. De Sousa RF, Chakravarty B, Sharma A, Parwaz MA, Malik A. Efficacy of triple therapy in auricular keloids. J Cutan Aesthet Surg. 2014;7(2):98–102.
117. Hatamipour E, Mehrabi S, Hatamipour M, Ghafarian Shirazi HR. Effects of combined intralesional 5-fuorouracil and topical silicone in prevention of keloids: a double blind randomized clinical trial study. Acta Med Iran. 2011;49(3):127–30.
118. Tanaydin V, Colla C, Piatkowski A, Beugels J, Hendrix N, Van Den Kerckhove E, et al. Management of ear keloids using custom-molded pressure clips: a preliminary study. Eur J Plast Surg. 2014;37(5):259–66.
119. Wang CJ, Ko JY, Chou WY, Cheng JH, Kuo YR. Extracorporeal shockwave therapy for treatment of keloid scars. Wound Repair Regen. 2018;26(1):69–76.
120. Kim DH, Han SH, Suh HS, Choi YS. Benefits of extracorporeal shock waves for keloid treatment: a pilot study. Dermatol Ther. 2020;33(4):e13653.
121. Tan C, Yeo Chen Long D, Cao T, Tan Wei Ding V, Srivastava R, Yow AP, et al. Drug-free microneedles in the treatment of keloids: a single-blinded intraindividual controlled clinical trial. Br J Dermatol. 2018;179(6):1418–9.
122. Bhusari P, Shukla J, Kumar M, Vatsa R, Chhabra A, Palarwar K, et al. Noninvasive treatment of keloid using customized re-188 skin patch. Dermatol Ther. 2017;30(5):e12515.
123. Weshay AH, Abdel Hay RM, Sayed K, El Hawary MS, Nour-Edin F. Combination of radiofrequency and intralesional steroids in the treatment of keloids: a pilot study. Dermatol Surg. 2015;41(6):731–5.
124. Berman B, Garikaparthi S, Smith E, Newburger J. A novel hydrogel scaffold for the prevention or reduction of the recurrence of keloid scars postsurgical excision. J Am Acad Dermatol. 2013;69(5):828–30.
125. Garakaparthi S. E-matrix injections for the revision of keloid scars: a novel treatment for the management of keloids. West Indian Med J. 2016;65:69.
126. Limthanakul I, Chatdokmaiprai C. Comparison between the efficacy of 5% imiquimod cream and intralesional triamcinolone acetonide in the prevention of recurrence of excised ear keloid: a prospectiverandomized study. J Med Assoc Thail. 2020;103(5):423–7.
127. Salunke A, Nakanekar A, Lahankar M, Kolpe H, Borkundwar S. A clinical study for the management of ear pinna keloid by ksharsutra and agnikarma. Int J Res Ayurveda Pharmacy. 2014;5(3):261–5.
128. Sigler A. Use of colchicine to prevent recurrence of ear keloids. A new approach. J Plast Reconstr Aesthet Surg. 2010;63(8):e650–2.
129. Song KX, Liu S, Zhang MZ, Liang WZ, Liu H, Dong XH, et al. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J Zhejiang Univ Sci B.
2018;19(11):853–62.
130. Gold MH, Berman B, Clementoni MT, Gauglitz GG, Nahai F, Murcia C. Updated international clinical recommendations on scar management: part 1--evaluating the evidence. Dermatol Surg. 2014;40(8):817–24.
131. Ogawa R, Akita S, Akaishi S, Aramaki-Hattori N, Dohi T, Hayashi T, et al. Diagnosis and treatment of keloids and hypertrophic scars-Japan scar workshop consensus document 2018. Burns Trauma. 2019;7:39.
132. Juckett G, Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80(3):253–60.
133. Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, et al. Updated international clinical recommendations on scar management: part 2--algorithms for scar prevention and treatment. Dermatol Surg.2014;40(8):825–31.
134. Lv K, Xia Z. Chinese consensus panel on the p, treatment of s. Chinese expert consensus on clinical prevention and treatment of scar(). Burns. Trauma. 2018;6:27.
135. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43. Published 2010 Jun 21.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the Walsh et al. Systematic Reviews (2023) 12:42 by Wound World.


