Moisture-associated skin damage (MASD) is a complex and commonly recognised condition. Overexposure of the skin to bodily fluids can compromise its integrity and barrier function, making it more permeable and susceptible to damage (Gray et al, 2011; Woo et al, 2017). Individuals with MASD experience persistent symptoms that affect quality of life, including pain, burning and pruritis (Gray et al, 2011; Woo et al, 2017).
When the skin becomes too moist, it undergoes a process of maceration; this causes the skin to soften and break down. The connective fibres can be teased apart, and the skin often exhibits a white appearance.
Diabetes-related foot ulcers
Diabetes-related foot ulcers (DRFUs) represent a major global health concern, affecting up to 25% of people with diabetes mellitus (DM). These ulcers not only reduce the quality of life and physical functioning of those affected but also have a significant economic impact on communities and the health care system. Prompt and appropriate wound assessment informs optimal DRFU management and can prevent the development of severe infection and consequent lower-extremity amputation (Rowledge et al, 2016).
Periwound skin is subject to damage caused by adhesive dressing removal, excess, uncontrolled wound exudate, and pressure-induced callus formation. An impaired periwound can exacerbate pain and contribute to protracted healing. It appears wound healing is impacted by several specific periwound impairments such as overhydration, dryness and lipid deficiency.
Diabetic foot ulceration continues to be synonymous with delayed healing, higher infection rates and an increased risk of lower extremity amputation (Frykberg, 1998). Maceration of the wound bed and surrounding skin in diabetic foot ulceration may be one of the least well-recognised factors contributing to impaired healing (Cullum et al, 2000). The impact of maceration on skin integrity, and its traditionally poor management and frequency, make it an obvious contender for inclusion as a risk factor in wound care (Cutting and White, 2002b). However, there is Iittle research on the possible implications of maceration in diabetic foot ulceration (Bale et al, 2001).
Periwound maceration, a type of MASD, has been defined as the softening and breaking down of skin, resulting from prolonged exposure to moisture (Bowser and White, 1985).
Maceration of the epithelium has been described as softening of the skin by exposure due to excessive amounts of liquid for extended periods (Bowser and White, 1985). Maceration reduces the physical and chemical integrity of the stratum corneum, thereby predisposing the periwound area to potential invasion by bacteria and fungi (Butcher, 2000).
In the diabetic foot, loss of autonomic nerve supply can alter the vascular perfusion and nerve supply of the skin. This affects the integrity of the skin and its resistance to mechanical and chemical trauma from pressure and wound exudate (Faber et al, 1993). Consideration should be given to the effects of the water content in wound exudate, and to the degree and nature of the inflammatory exudate in acute and chronic wounds (Cutting and White, 2002a). It is also essential to include the periwound margins as an integral part of wound assessment (Cutting, 2001).
Skin
Overhydrated, macerated periwound skin can increase the risk of infection, precipitate inflammation, and result in wound enlargement. Studies demonstrate that maceration is a risk factor for the development of pressure injuries and that excessively moist skin is more susceptible to breakdown when subjected to compressive and shearing forces (Chen et al, 1992). Neuropathic DRFUs are commonly bordered by a macerated callus, which may be detrimental to wound healing, acting as a reservoir for bacteria and restricting drainage of wound exudate. Dry periwound skin may also impair healing, as dry skin is relatively inelastic and more susceptible to breakdown. It is less likely to withstand physical forces and can crack and fissure.
Skin dryness is commonly observed in people with diabetes as nerve changes can affect sudomotor function, decreasing sweat gland activity. When skin lipid levels are low, skin barrier function is compromised, which results in decreased ability to maintain optimal skin hydration and increased susceptibility to infection. In vitro testing has demonstrated that skin lipids play a role in the innate immune defence against bacterial colonisation and infection. There is a substantial delay in wound healing in patients with DM related to several abnormalities, such as decreased concentration of growth factors, increased protease activity, abnormalities of the extracellular matrix-reduced fibroblast function and impaired nutritive function (Gazzaruso et al, 2012).
The pigment melanin contributes to skin colour. Evidence suggests that the epidermal barrier is stronger and more rapidly repaired in dark pigmented skin with higher concentrations of melanin. It is hypothesised that melanin can inhibit the proliferation of infectious organisms of the skin through interaction with microbial peptides. Given that melanin has a role in the body’s immune defence system, periwound melanin levels may influence wound healing. Haemoglobin levels also contribute to skin colour. An increase in capillary blood flow and subsequent rise in haemoglobin concentration results in skin redness or erythema (Brenner and Hearing, 2008).
Wound exudate
Much effort has gone into ascertaining the best clinical practice to support wound treatment and, in past decades, moist wound healing has become the accepted practice for treating acute wounds and has been extended to treating chronic wounds. A key aspect in the management of diabetic foot ulcers (DFUs) is maintaining a wound environment that optimises healing.
Wound exudate is a generic term given to liquid produced from wounds, fistulae, and other more acute injuries once haemostasis has been achieved (Thomas, 1997). Exudate keeps the wound moist, supplies nutrients, and provides the medium for migration and mitosis of epithelial cells. This, in turn, keeps the wound supplied with leucocytes and helps to control bacteria (Quick, 1994). However, the optimum moisture content in a wound has not been established— excessive exudate can cause maceration and excoriation of the surrounding skin possibly resulting in more pain and trauma, which increases the size of the original ulcer and prolongs the healing process (Nelson, 1997).
For the diabetic foot, dressings should be non-adherent, not too bulky and able to absorb exudate and withstand pressure if the patient is ambulatory (Foster et al, 1994). There are many products currently available with claims of beneficial properties. Practitioners should keep up to date with developments in wound care and be conversant with the research that supports or refutes the claims made by manufacturers (Moody, 2000). This is even more important considering clinical governance. The practitioner should be certain that the care they give is clinically effective and evidence-based (Cullum, 1998).
Effective management of wound exudate in DFUs is a key component of any wound management plan and crucial to facilitate healing. Clinicians should recognise when exudate has become detrimental in the healing process. If maceration still occurs, it may be necessary to increase dressing changes to prevent the destructive effect of exudate on periwound margins. Sound clinical judgment is necessary to ensure that the balance between hydration of slough at the wound bed is not at the expense of destruction of surrounding tissue.
A high number of reviews within the existing body of knowledge discuss maceration of the skin and wound. Nevertheless, these are based on laboratory studies and not human subjects and, while the biomedical research is invaluable in terms of how moisture affects the epidermis, it is important to look at the clinical research about how maceration affects the patient. The National Institute of Health and Care Excellence (NICE) guidelines on chronic wounds highlight the lack of high-quality research that can inform clinical practice (NICE, 2016).
Various studies use maceration as an outcome measure of the role of wound dressings in retaining exudate away from the healthy skin. However, few have examined the effects of maceration on the diabetic foot (Bale et al, 2001). There is a need for further research into the appropriate use of dressings and their role in reducing maceration in diabetic foot lesions.
Assessment and prevention strategies are of key importance in all types of MASD, including maceration. Interventions to protect the skin and prevent MASD include the use of skin protection products, such as barrier creams, liquid polymers and cyanoacrylates to create a protective layer on the skin surface that simultaneously maintains hydration levels while blocking external moisture and irritants (Gray et al, 2011; McNichol et al, 2018).
Emerging evidence now highlights the links between MASD and other skin conditions such as cutaneous infection and pressure ulcers (PUs; Jones et al, 2008; Beeckman et al, 2014). Adopting a holistic, integrated approach that focuses on prevention strategies and the importance of skin integrity can have overall beneficial results and help to break down barriers to effective care in practice (Beeckman et al, 2020).
Clinicians must be vigilant, both in maintaining optimal skin conditions and diagnosing and treating early stages of MASD to prevent progression and skin breakdown (Gray et al, 2011). Maceration itself appears to be ill-defined and a consensual definition of what constitutes skin maceration and how to measure it in clinical settings is lacking, which may impact on patient care. Before maceration can be managed or prevented effectively, it is important to be able to identify and measure it, as well as recognise the warning signs that are the precursors to maceration. There is no guidance on the critical time between a healthy moisture level and maceration. Guidance on this would be useful in clinical practice to be able to prevent maceration, rather than managing it once it has already occurred.
The skin surrounding a wound is particularly vulnerable and, although it may appear healthy, periwound complications frequently occur (Bianchi, 2012). According to Hunter et al (2013), the integrity of the periwound skin may be an important determinant in decreasing wound size. There are several wound-related factors that may result in periwound damage, such as exposure to exudate and the matrix metalloproteinases that exudate contains, infection, dressing adherence or allergic reactions (Bianchi, 2012). Types of periwound damage include maceration denudement, excoriation, erosion, skin stripping and allergic reactions affecting the skin. Skin irritation can also lead to excoriation (Bianchi, 2012); pruritus (itchy skin) may also occur, leading to further issues.
Wound cleansing is defined as the removal of surface contaminants, bacteria and remnants of previous dressings/ treatments from the wound surface and its surrounding skin (Rodeheaver and Ratliff, 2018).
Periwound complications can delay healing in a variety of ways, increasing the risk of infection; increased bacterial burden can in turn increase inflammatory response and delay healing, creating a vicious cycle. Healthcare costs increase when periwound complications develop such as delayed healing, increased wound size and increased pain. Additional care time, careful monitoring, and ongoing assessment of both the wound and periwound skin can aid in identifying skin changes and ensuring early intervention with appropriate and cost-effective treatment options (Bianchi, 2012). Any individual with a wound may develop periwound complications, but individuals with fragile skin are more susceptible. Additional risk factors may increase an individual’s likelihood of developing these complications. It is important to note that, while measures should be taken to mitigate risk, not aIl periwound skin damage is avoidable.
Skin breakdown, erythema and erosion commonly occur in skin that has been damaged by wound exudate (Bianchi, 2012). Maceration may also occur when moisture is trapped against the skin for a prolonged period. This may appear as a white margin around the wound, causing the skin to soften and wrinkle (Lawton and Langøen, 2009). Maceration may increase risk of friction damage, infection, and skin breakdown, resulting in enlargement of the wound or delayed healing (Colwell et al, 2011).
While the production of exudate is vital to the wound healing process, if not managed effectively, exudate can cause damage to the periwound (surrounding) skin (World Union of Wound Healing Societies [WUWHS], 2019). The prevalence of periwound maceration is not well documented, but it is acknowledged that its impact is ‘substantial’, both on individuals and healthcare systems (Woo et al, 2017).
Managing periwound maceration
Periwound maceration delays overall wound healing and is also correlated with higher pain levels prior to and during dressing changes (Woo et al, 2017). The use of an acrylate terpolymer barrier film has been found to facilitate the healing of larger wounds without increasing costs; hence, use of an acrylate terpolymer barrier film for periwound skin protection in patients with exuding ulcers is recommended (Guest et al, 2012).
To manage periwound maceration, the cause of excess exudate should be identified. Any management strategy must then address the factors that are contributing to high exudate levels and potential periwound damage, as well as physically handling the volume of exudate. Any contributing patient comorbidities, medications or psychosocial factors should also be addressed.
Management of moisture and consideration of microclimate may encompass:
- Identifying the cause and systemic management of the type/amount of wound exudate plus underlying causes: increased with venous disease due to venous pooling, infection, fluid overload (heart failure, liver, or kidney disease), decreased in individuals with circulation problems
- Considering humidity: increase airflow, consider occlusive dressings, clothing, fabric types, and incontinence management
- Considering temperature (relating to the individual and the environment): positioning, comorbidities/ medications
- Considering airflow: may be affected by the location of the wound, mattress type (high air loss versus low air, or standard mattress or foam; mattress covers may be used), or type of footwear in patients with a DFU
- Selecting dressings/bandages/devices that adequately address local wound conditions (fluidhandling capacity); materials that lock fluid or minimise lateral movement, amount of compression and dressing moisture vapour transmission rate.
Changing and improving practices for microclimate management requires a multipronged approach. Newer technologies should be explored to improve traditional practices and reduce potential risk factors around skin damage due to microclimate. An evidence-based approach combined with education may lead to improved skin integrity in at-risk individuals.
Wound cleansing is defined as the “removal of surface contaminants, bacteria and remnants of previous dressings/treatments from the wound surface and its surrounding skin” (Rodeheaver and Ratliff, 2018). In any wound cleansing routine, the sequence of cleansing — the wound, the periwound and, for example, the limb — is important. The surrounding skin should be cleansed thoroughly but gently, without causing undue trauma. A vigorous rubbing technique should be avoided. Once the dressing is removed, a pack (a sterile gauze moistened with a cleansing solution to prepare for the wound cleansing) can be placed on the wound while the periwound and/or limb is cleansed with a skin-friendly cleanser, using disposable moistened cloths or commercial cleansing pads.
When caring for an individual with a wound, healthcare professionals should assess the periwound at dressing changes, planning management according to the changing risk factors identified.
It is important when protecting the periwound from moisture to (adapted from Colwell et al, 2017):
- Select a primary dressing with wound-appropriate absorptive properties, or use a secondary dressing
- Consider the size of the wound when selecting the size of the absorbent dressing
- Apply a liquid barrier film (e.g. protective barrier film or cyanoacrylate) to cover the area under the dressing (see instructions for use below)
- Plan dressing change frequency according to the amount and type of wound exudate
- Remove dressings slowly — sterile water/saline soaks or a medical adhesive remover may be required if the non-adhesive dressing is adhered to the wound edge and/or periwound skin
- Use ‘picture frame’ method, as used for peristomal skin irritation
- Apply stoma powder to absorb excess moisture or use the ‘crusting technique’ according to local guidance, where appropriate.
The risk of skin trauma during dressing/device removal should be minimised (WUWHS, 2019). Use of low-adherent or silicone dressings, tapes or securement devices, and application of periwound skin protectant ointments, creams or barrier films may help to protect the skin and reduce the risk of damaging the skin further (Bianchi, 2012).
Skin protection products should be used to protect the periwound skin. Advanced polymer-based barriers can be used where exudate levels are very high, or where dressing wear time may be extended beyond control. Film-forming barriers may also be considered as part of a treatment regimen where large expanses of adhesive are used and replaced frequently, such as with negative pressure wound therapy.
Skin protection products should always be used according to the manufacturer’s instructions and based on suitability for the patient and their wound. For instance, some skin protection products may interfere with dressing adherence and absorption and,
therefore, should only be used on suitable wounds (WUWHS, 2019).
A multidisciplinary team approach for managing periwound maceration in DRFUs
Many DRFUs in Europe are overseen by podiatrists who make the clinical decision to refer the patient to the full MDT. Some countries stipulate that patients are managed by physicians who make the decisions on the care plan and referrals. Where possible clinically, a PU or DRFU should be managed to ensure timely ulcer closure. Standards of care specific to the management of a PU and DRFU have been published (Bus et al, 2015; Saskatchewan Ministry of Health, 2016; Ibrahim, 2017; NICE, 2017). However, where available, local guidelines should be followed.
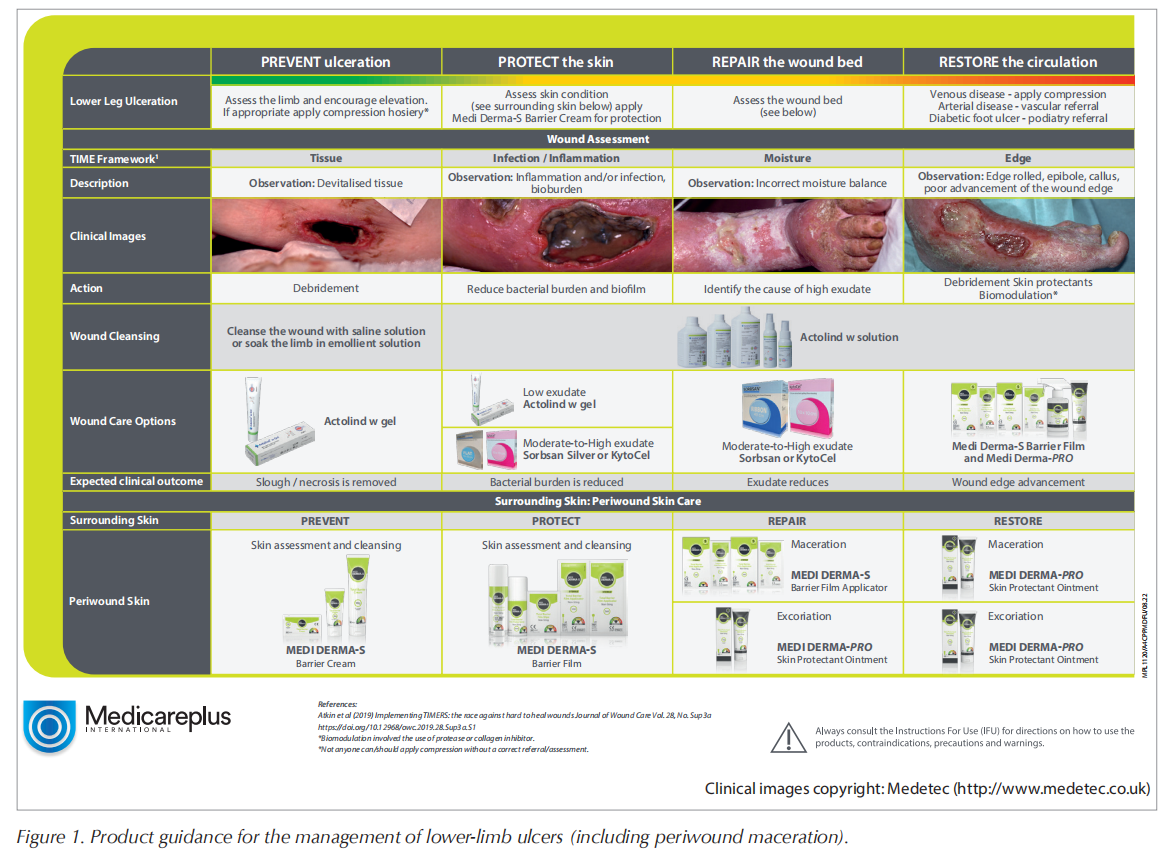
There are also several generic guidelines on principles of best practice in wound management, for example, the TIME framework is referenced frequently and used widely (Sibbald et al, 2011; Dissemond et al, 2015; Harries et al, 2016). TIME is a global wound care framework used to implement appropriate care plans and promote wound healing and can be used to apply wound bed preparation to practice. Correct implementation depends on patient and wound assessment.
TIME is not a sequential process as every wound is different; simultaneous attention to one or more of the components may be required. The principles of the TIME framework are used to guide health professionals on what to assess and treat in the wound bed to aid healing.
The TIME framework involves removing barriers to wound healing:
T Tissue management: viable, non-viable, deficient (wound cleansing and irrigation, e.g. Actolind® w Solution, Actolind® w Gel [marketed by Medicareplus International])
I Infection and inflammation control (wound dressing with antimicrobial and bacteriostatic properties, e.g. Sorbsan Silver, KytoCel [marketed by Medicareplus International])
M Moisture balance (exudate management, e.g. Sorbsan, KytoCel [marketed by Medicareplus International])
E Epidermal margin: non-advancing or undermined (create barrier to prevent or protect surrounding skin, e.g. Medi Derma-S Barrier Cream [Medicareplus International], Medi Derma-S Barrier Film Range [Medicareplus International], as well as restore when there is periwound damage, e.g. Medi Derma-PRO Skin Protectant ointment [Medicareplus International]).
Cleansing should be done at each dressing change and wound assessment, and prior to the application of a new dressing. Cleansing of the periwound skin and surrounding skin allows for visualisation and management of tissue surrounding the ulcer (e.g. with Medi Derma-PRO Cleanser [Medicareplus International]). Managing moisture balance can be challenging in patients with DRFUs or venous leg ulcers (Moffatt et al, 2009). Excessive drainage should be managed to prevent periwound maceration, wound extension or hypergranulation.
Medi Derma-S Total Barrier Film Range
Medi Derma-S Total Barrier Film is a non-sting medical barrier film range that offers maximum barrier protection (up to 72 hours) from MASD on intact and moderately damaged skin. Medi Derma-S Total Barrier Film is available in 5 formats (bag-on-valve aerosol film spray, pump spray, film wipes and 1ml and 3ml film applicators) for all wound types and sizes. See Box 1 for benefits of Medi Derma-S Total Barrier Film range.
Its application helps to prevent irritation and skin breakdown from incontinence, other bodily fluids (sweat, wound exudate, stoma leakage), adhesive products and friction. It is ideal for skin folds, periwound, peristomal and intravenous site protection.
Instructions for use:
Medi Derma-S Total Barrier Film Spray is supplied in a 30ml pump spray and 50ml bag-on-valve aerosol spray. The ease of use with the bag-on-valve spray is that it can be sprayed and used at any angle (360°). It is quick and easy to use and ideal for sore skin-no touch application.
Medi Derma-S Total Barrier Film Applicator is a sterile device designed to provide a transparent, quick drying and long-lasting barrier protection. Medi Derma-S Total Barrier Film Applicators are supplied in two sizes, 1ml and 3ml. The product is sterile and must not be used if the packaging is damaged or open.
Medi Derma-S Total Barrier Film Wipes is a sterile device designed in convenient, discreet, individual sachets for quick and easy use.
Conclusion
Maceration affects the skin at a greater depth than previously thought, which may have implications in terms of treatment and healing times. It causes discomfort and pain to patients and prolongs healing times.
Moisture that macerates the skin takes various forms including perspiration, wound exudate, urine, faeces, mucus, and saliva, with implications for clinical management. It is a severe condition that merits more focused research and exploration in drawing water content out of wounds and away from the skin to maintain a healthy, balanced environment.
Clinicians play an important role in ensuring safe practice standards are observed and that care delivered is evidence-based. Education is one of the key steps in ulcer prevention and the clinical practice followed by healthcare professionals must be supported by evidence and best practice. All staff should be educated on best practices and care pathways must reflect the evidence, such as making sure that checklists and assessments include the periwound skin as a part of routine wound care.
REFERENCES
1. Bale S, Baker N, Crook H et al (2001) Exploring the use of an alginate dressing for diabetic foot ulcers. J Wound Care 10(3): 81–4
2. Beeckman D, Van Lancker A, Van Hecke A, Verhaeghe S (2014) A systematic review and meta-analysis of incontinence- associated dermatitis, incontinence, and moisture as risk factors for pressure ulcer development. Res Nurs Health 37(3): 204–18
3. Beeckman D, Campbell K, Le Blanc K et al (2020) Best practice recommendations for holistic strategies to promote and maintain skin integrity. Wounds International, London. Available at: www.woundsinternational.com
4. Bianchi J (2012) Protecting the integrity of the periwound skin. Wound Essentials 1: 58–64
5. Bowser PA, White RQ (1985) Isolation, barrier properties and lipid analysis of stratum compactum, a discrete region of the stratum corneum. Br J Dermatol 112(1): 1–14
6. Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin. Photochem Photobiol 84(3): 539–49
7. Bus SA, van Netten JJ, Lavery LA et al (2015) IWGDF Guidance on the prevention of foot ulcers in atrisk patients with diabetes. Available at: https:// tinyurl.com/33ruhybt (accessed 11.07.2022)
8. Butcher M (2000) The management of skin maceration. Nursing Times 96(45): 35–36
9. Chen WY, Rogers AA, Lydon MJ (1992) Characterization of biologic properties of wound fluid collected during early stages of wound healing. J Invest Dermatol 99(5): 559–64
10. Colwell JC, McNichol L, Boarini J (2017) North America Wound, Ostomy, and Continence and Enterostomal Therapy Nurses Current Ostomy Care Practice Related to Peristomal Skin Issues. J Wound Ostomy Continence Nurs 44(3): 257–61
11. Colwell JC, Ratliff CR, Goldberg M et al (2011) MASD part 3: peristomal moisture- associated dermatitis and periwound moisture-associated dermatitis: a consensus. J Wound Ostomy Continence Nurs 38(5): 541–53; quiz 554–5
12. Copson D, Freitas A (2021) A multi-centred retrospective analysis of 336 clinical evaluations of the Medi Derma Total Barrier Protection (TBP™) Product Range. Wounds UK 17(1): 89–97
13. Cullum N (1998) Clinical effectiveness in nursing. Nursing Times Research 3(1): 15–18
14. Cullum N, Najid M, O’Meara S, Sheldon T (2000) Use of dressings: is there an evidence base? In: Boulton ARM, Connor H, Cavanagh PR (Eds.) The Foot in Diabetes 3rd Edn. Wiley, Chichester. 153–68
15. Cutting KF (2001) The causes and prevention of maceration of the skin. Prof Nurse 17(3): 177–8
16. Cutting KF, White RJ (2002a) Avoidance and management of peri-wound maceration of the skin. Prof Nurse 18(1): 33, 35–36
17. Cutting KF, White RJ (2002b) Maceration of the skin and wound bed. 1: Its nature and causes. J Wound Care 11(7): 275–8
18. Dissemond J, Kröger K, Storck M et al (2015) Topical oxygen wound therapies for chronic wounds: a review. J Wound Care 24(2): 53–4, 56–60, 62–3
19. Faber WR, Michels PPJ, Naafs B (1993) The neuropathic foot. In: Westerhof W (Ed) Leg ulcers diagnosis and treatment Elsevier, Amsterdam Chapter 10
20. Foster A, Greenhill M, Edmonds M (1994) Comparing two dressings in the treatment of diabetic foot ulcers. J Wound Care 3(5): 224–8
21. Frykberg R (1998) Diabetic foot ulcers: current concepts. J Foot Ankle Surg 37(5): 440–6
22. Gazzaruso C, Coppola A, Montalcini T et al (2012) Lipoprotein(a) and homocysteine as genetic risk factors for vascular and neuropathic diabetic foot in type 2 diabetes mellitus. Endocrine 41(1): 89–95
23. Gray M, Black JM, Baharestani MM et al (2011) Moisture-associated skin damage: overview and pathophysiology. J Wound Ostomy Continence Nurs 38(3): 233–41
24. Guest JF, Taylor RR, Vowden K, Vowden P (2012) Relative cost-effectiveness of a skin protectant in managing venous leg ulcers in the UK. J Wound Care 21(8): 389–94, 396–8
25. Harries RL, Bosanquet DC, Harding KG (2016) Wound bed preparation: TIME for an update. Int Wound J 13(Suppl 3): 8–14
26. Hunter SM, Langemo D, Thompson P et al (2013) Observations of periwound skin protection in venous ulcers: a comparison of treatments. Adv Skin Wound Care 26(2):62–6
27. Ibrahim A (2017) IDF Clinical Practice Recommendation on the Diabetic Foot: A guide for healthcare professionals. Diabetes Res Clin Pract 127: 285–87
28. Jones JE, Robinson J, Barr W, Carlisle C (2008) Impact of exudate and odour from chronic venous leg ulceration. Nurs Stand 22(45): 53–4, 56, 58 passim
29. Kamal K, Powell RJ, Sumpio BE (1996) The pathobiology of diabetes mellitus: implications for surgeons. J Am Coll Surg 183(3): 271–89
30. Lawton S, Langøen A (2009) Assessing and managing vulnerable periwound skin. Available at: https://tinyurl.com/6mu7xmyw (accessed 11.07.2022)
31. McNichol LL, Ayello EA, Phearman LA et al (2018) Incontinence-Associated Dermatitis: State of the Science and Knowledge Translation. Adv Skin Wound Care 31(11): 502–13
32. Moffatt C, Kommala D, Dourdin N, Choe Y (2009) Venous leg ulcers: patient concordance with compression therapy and its impact on healing and prevention of recurrence. Int Wound J 6(5): 386–93
33. Moody M (2000) Principles of wound management. Nursing and Residential Care 1(4): 229–32
34. Moore Z, Butcher G, Corbett LQ et al (2014) Exploring the concept of a team approach to wound care: Managing wounds as a team. J Wound Care 23 Suppl 5b: S1–S38
35. National Institute for Health and Care Excellence (2016) Chronic wounds: advanced wound dressings and antimicrobial dressings. Available at: https://tinyurl.com/3wfmyvcn (accessed 11.07.2022)
36. National Institute for Health and Care Excellence (2017) Preventing pressure ulcers in adults. Available at: www.nice.org.uk (accessed 11.07.2022)
37. Nelson A (1997) Is exudate a clinical problem? In: Proceedings, joint meeting, European Wound management Association and European Tissue Repair Society: management of wound exudate. Cherry G, Harding K (Eds) Churchill Communications, London
38. Quick A (1994) Dressing choices. Nursing Times 90(45): 71–2
39. Rodeheaver GT, Ratliff CR (2018) Wound cleansing, wound irrigation, wound disinfection. In: Krasner DL, van Rijswijk L (Eds.) Chronic Wound Care: The Essentials
40. Rowledge A, Frescos N, Miller C et al (2016) The diabetic foot ulcer periwound: a comparison of visual skin assessment and a skin diagnostic device. Wound Practice and Research 24(3): 160–8
41. Saskatchewan Ministry of Health (2016) Clinical practice guidelines for the prevention and management of diabetes foot complications. Available at: https://tinyurl.com/23ta2j85 (accessed 11.07.2022)
42. Sibbald RG, Goodman L, Woo KY et al (2011) Special considerations in wound bed preparation 2011: an update©. Adv Skin Wound Care 24(9): 415–36; quiz 437–8
43. Thomas S (1997) Assessment and management of wound exudate. J Wound Care 6(7): 327–30
44. Woo KY, Beeckman D, Chakravarthy D (2017) Management of Moisture-Associated Skin Damage: A Scoping Review. Adv Skin Wound Care 30(11): 494–501
45. World Union of Wound Healing Societies (2019) Wound exudate: Effective assessment and management. management. London: Wounds International. Available at: www.woundsinternational.com
This article is excerpted from the 《The Diabetic Foot Journal Vol 25 No 3 2022》by Wound World.


