Diabetic wounds, particularly in the lower extremities, represent a serious complication, with severe consequences in terms of morbidity, mortality and health cost burden (Boulton et al, 2005). Conventional treatment of chronic diabetic wounds entails regular debridement and dressing changes (McCartan and Dinh, 2012). The availability of skin grafting techniques using tissue flaps, with or without skin grafting and skin substitutes, has broadened the range of treatment options and enabled reconstructive surgery as an alternative modality for complicated wounds.
A split-thickness skin graft (STSG) is one such procedure that has been used successfully to close challenging wounds (e.g. diabetic foot and ankle wounds) once a granular base is achieved and where the wound is relatively large but cosmetic appearance is not a major concern (Ramanujam et al, 2010; Grande and Elson, 2021). Besides good granulation tissue, the wound bed must also be free of infection and well vascularised (Blair and Brown, 1968). When used for primary closure on optimised diabetic foot wounds, STSGs achieved a 78% success rate (90% wound closure) within 8 weeks (McCartan and Dinh, 2012). More recently, a meta-analysis of 11 studies on STSGs for diabetic foot ulcers estimated a healing rate of 85.5%, with a mean healing time of 5.4 weeks, and low rates of recurrence (4.2%; Yammine and Assi, 2019). These compare favourably with estimates reported for conventional management, where 24–47% of wounds took 12 weeks to heal and 31–68% took up to 20 weeks (Margolis et al, 1999).
Key parameters for successful graft uptake are the matching of the graft size to that of the recipient wound site, and application of even pressure on the graft from the dressing used to secure the site (Blair and Brown, 1968). Available methods for securing the graft site include tie-over dressings, bolster dressings, sterilised rubber bands, negative pressure dressings and fibrin glues. Although there is no consensus on the optimal method, most evidence supports the use of simple pressure dressings and quilting/mattress suturing (Ogawa et al, 2007; Joyce et al, 2015; Kromka et al, 2018; Steele et al, 2020). Bolster dressings can support and cushion the grafts and enhance graft survival (Adams et al, 2004).
In our practice, we have often used simple tie-over dressings with long silk sutures to secure the bolster to the recipient site. However, this procedure is time-consuming and it can be complicated to secure the suture ends when changing the pressure dressing.
Alternatively, the use of semipermeable polyurethane foam dressings has been proposed to secure wound sites, due to their non-occlusive nature, ability to maintain a moist environment for wound healing and overall tolerability. Published studies on foam dressings, with or without added antimicrobial agents, have reported applications in STSG wound management, although reports on the use of polyurethane foam dressings in STSGs are mostly for donor site management (Park et al, 2002; Wiechula, 2003; Brown and Holloway, 2018; Pak et al, 2019).
In the published literature, only one report was identified on the use of foam dressings for securing skin grafts at the recipient site, which was a case series of 25 patients with wounds ranging in size from 6.0 cm × 4.5 cm to 10.0 cm × 8.0 cm (Sakurai et al, 2007). The authors reported good results with the use of two layers of polyurethane foam and film as a wound dressing, including ease of use and rapid healing.
BETAplast Silver
BETAplast™ Silver (Mundipharma) belongs to a range of three-layer polyurethane foam dressings that comprise a protective semipermeable outer layer, a hydrophilic middle layer with a high fluid absorption/retention capacity to reduce maceration, and a wound contact layer with a micropore structure called SMARTPORE Technology. SMARTPORE Technology is designed to prevent tissue ingrowth during wound healing (Lee et al, 2016a; 2016b; 2018). The small pore size of the wound contact layer (Lee et al, 2016b; 2018) reduces ingrowth of fibroblasts and epithelial cells, leading to less pain at dressing changes.
Similar polyurethane foam dressings utilising SMARTPORE Technology (Medifoam; Genewel) were found to support more rapid STSG donor site wound healing than hydrocellular dressings, with less pain and greater ease of handling (Park et al, 2002). A povidone‐iodine-impregnated polyurethane foam dressing (Betafoam; Genewel) supported donor site healing with more rapid epithelialisation than either hydrocellular foam dressings or conventional petrolatum-gauze dressings (Pak et al, 2019).
In addition to the properties described above, the BETAplast Silver dressing used in this report also contains silver sulfadiazine to suppress bacterial growth and reduce the risk of wound infection, which may be beneficial in managing diabetic wounds.
Aim
The authors aimed to investigate the application of an advanced foam dressing (BETAplast Silver) for wound healing at the STSG recipient site in patients with diabetic wounds.
Methods
Data were retrieved from the case records of all patients with diabetic wounds who underwent STSG recipient site wound management with polyurethane foam dressing (BETAplast) at the Capitol Medical Center, the Philippines, from July 1, 2019 to July 1, 2020. Patients who had a history of allergy to foam dressings, or concomitant inflammatory disease, or who had also undergone other kinds of surgery during the observation period were excluded from this analysis. Patients provided informed consent for the surgical procedure, documentation of treatment for clinical decision-making, and use of data for education and research purposes.
STSG procedure
STSGs of 0.18mm thickness with a size comparable to the wound at the recipient site were harvested from the lateral aspect of the patient’s ipsilateral thigh using an electric-powered dermatome. Wound size (cm) was determined based on length/width measurements (“head-to-toe” along the longest dimension, and from side-to-side at the widest point perpendicular to the length). The harvested graft was manually perforated with a surgical blade. After wound bed preparation, the perforated STSG was secured to the recipient site with skin staplers at regular intervals. The foam dressing was laid over the STSG and secured in place with several layers of wadding sheets. Standard postoperative care included wound debridement as necessary and regular dressing changes.
Outcomes and data analysis
Outcomes for the required assessment time points were extracted from patients’ records. In accordance with the treating clinician’s standard practice for postoperative wound management, STSGs were assessed on the fifth postoperative day (POD 5) and at dressing changes up to POD 30.
The following outcomes were assessed to evaluate the performance of the foam dressing:
1. Ease of use of the foam dressing. “Easy to use” meant the clinician was able to perform the task quickly; “difficult to use” — the clinician had difficulties with the dressing; “complicated to use”
— the clinician struggled to use it.
2. Presence of skin and soft tissue infection using The Infectious Diseases Society of America (IDSA) Guidelines (Stevens et al, 2014).
3. Graft viability, measured as percentage of graft survival.
4. Presence of fluids, such as seroma or haematoma. Demographic data, clinical characteristics, and outcome parameters were summarised using descriptive statistics.
Results
Characteristics of patients and STSG recipient sites
The demographic and clinical characteristics of the patients are summarised in Table 1. The average age was 61.8 ± 11.5 years and over half (61.1%) of the patients were women. The average HbA1c was 7.4% (77.8% of patients had an HbA1c ≥6.5%). Of the 18 patients, 11 (61.1%) had diabetic foot ulcers and six (33.3%) had non-healing wounds; there was one case of necrotising fasciitis.
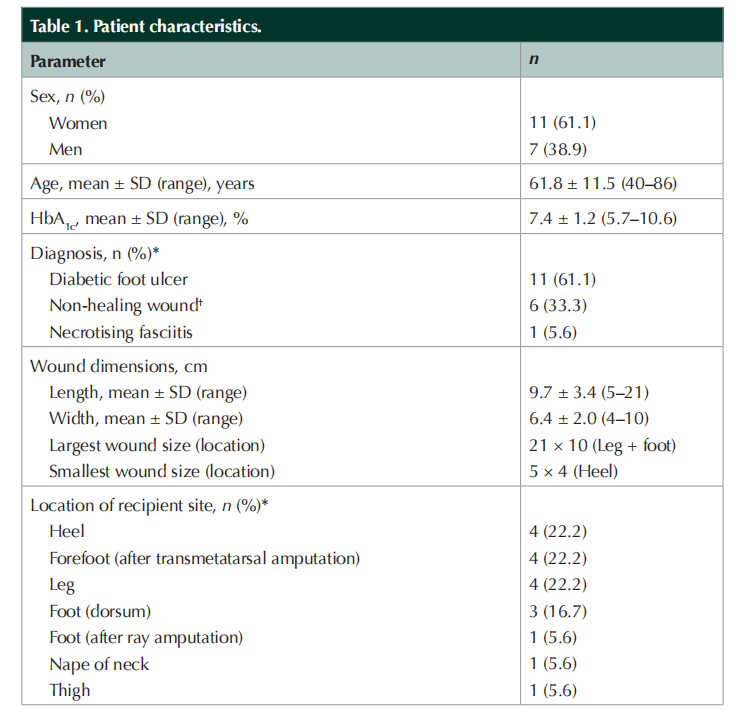
On average, wound dimensions were 9.7 cm ± 3.4 cm (length) and 6.4 cm ± 2.0 cm (width). Wound area ranged from 5 cm × 4 cm to 21 cm × 10 cm. Most STSG recipient sites were on the lower limbs (i.e. leg, heel and forefoot), including five post-amputation surgical wounds (Table 1). Some wounds were located in areas of the body that are particularly difficult to dress due to their anatomical location and/or mobility considerations. Figure 1 shows STSG wound management with the polyurethane foam dressing for three patients with diabetic wounds.
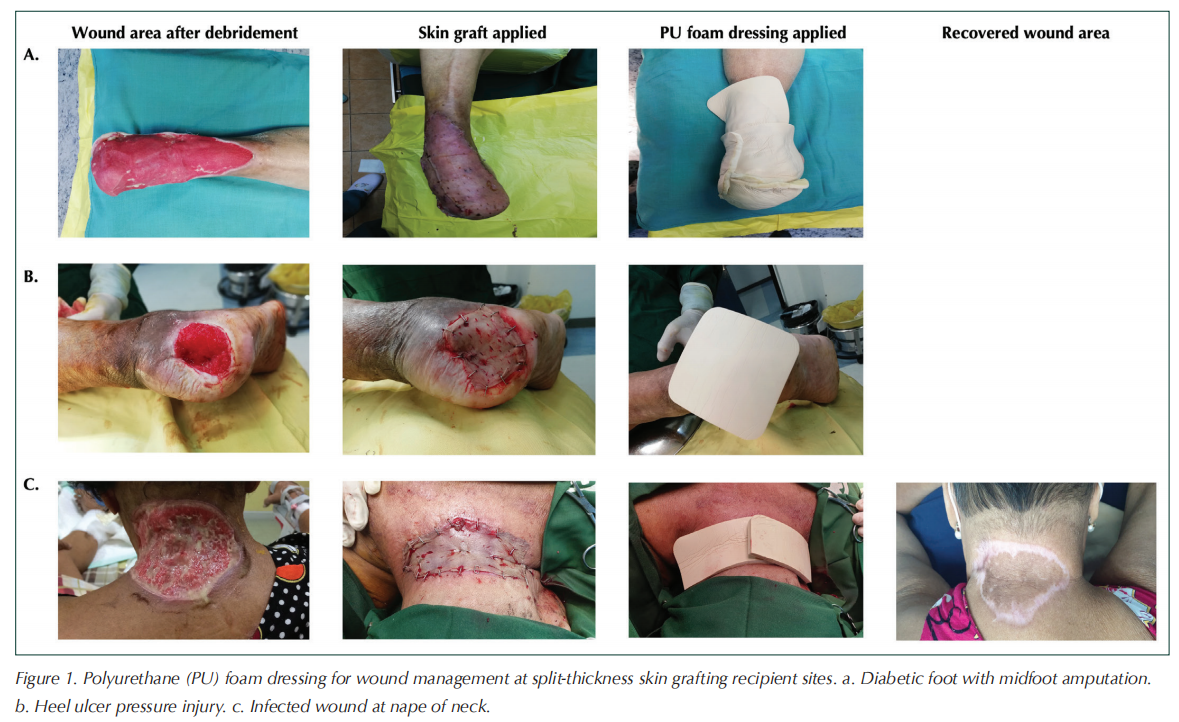
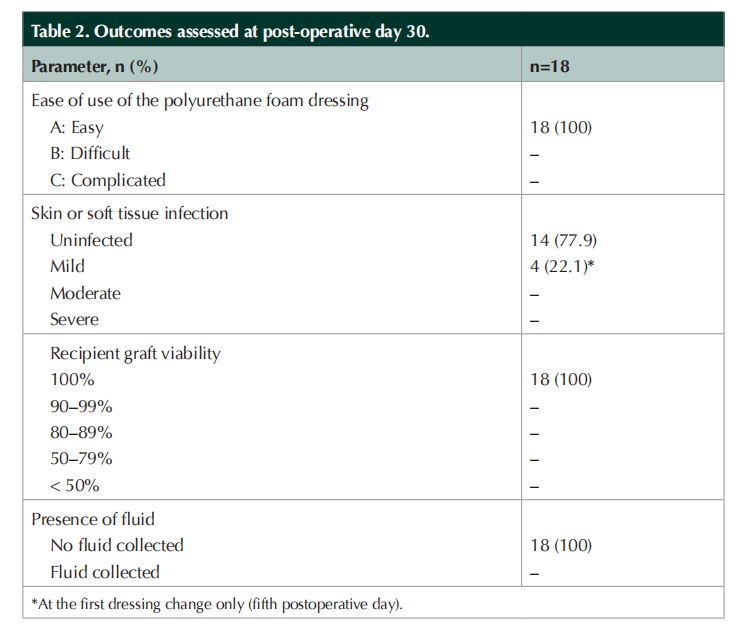
Wound healing outcomes and assessment of polyurethane foam dressing performance
Table 2 summarises the outcomes as assessed at POD 30. Across all STSG locations, the foam dressing was rated as “easy to use”. Graft uptake was 100% (n=18), with all STSGs being viable at POD 30. Most wounds remained free of infection throughout the 30-day observation period. In four patients, there were signs of mild infection at the first postoperative dressing change on POD 5, with wound cultures showing some organism growth. These rapidly resolved with the administration of antibiotics to which the organism was sensitive, with no further signs of infection at the recipient sites during subsequent dressing changes. None of the recipient site wounds showed signs of fluid accumulation at dressing changes over the 30-day observation period.
Discussion
Of the various approaches to diabetic wound management, STSGs combined with an appropriate postoperative wound management strategy has emerged as a favourable option for achieving foot and ankle wound closure in patients with diabetes. Wound management in patients with diabetes is challenging due to multiple factors, including poor vascular function, neuropathy, slow wound healing, and increased susceptibility to infection. In light of the considerations of promoting graft viability, reducing wound trauma, infection control and exudate management, the postoperative regimen, including the choice of wound dressing, is crucial to the success of STSGs for diabetic wounds.
The foam dressing used in these cases for STSG recipient site dressing is an advanced three-layer, hydrophilic, polyurethane foam dressing (BETAplast Silver), with improved exudate absorption and retention properties to reduce the risk of wound maceration. The small pore size of the wound contact layer minimises tissue ingrowth into the dressing, reducing trauma and supporting easy dressing removal during dressing changes (Lee et al, 2016a; 2016b). The dressing also contains silver sulfadiazine, an antimicrobial agent, which may help to prevent infection of problematic wounds such as diabetic ulcers or burns (Ha et al, 2007).
In these 18 cases, excellent graft uptake was observed, with 100% viability at POD 30. The overall rate of infection of wounds was low and only dour cases of mild infection were recorded throughout the 30-day observation period. These were detected at the first postoperative dressing change (POD 5), and readily resolved with appropriate antibiotic treatment. The dressing was assessed by clinicians as easy to use on a range of anatomical sites. It was also able to prevent fluid accumulation, indicating good exudate management.
Conclusion
Our clinical observations with STSG combined with the use of the foam dressing in these 18 patients with diabetes wounds are encouraging and warrant further exploration. Due to the retrospective nature of data collection from patient records, some parameters of interest, such as healing time, could not be obtained. Specifically designed and controlled studies could be conducted to obtain more precise measurements of wound healing parameters and other benefits of this wound care regimen. Based on our observations, use of an advanced polyurethane foam dressing has the potential to promote graft viability at the STSG recipient site with good infection control.
Acknowledgements
The author thanks the surgical residents of Capitol Medical Center and the staff at the Diabetic Foot and Wound Center for their assistance in caring for the patients and their role in this study. The author also thanks Mundipharma, Philippines for their continuous support.
Funding
No funding was received for this research. Funding for manuscript preparation and editorial support was provided by Mundipharma Singapore Holding Pte Ltd.
Disclosure
The author has no conflicts of interest to declare.
REFERENCES
1. Adams DC, Ramsey ML, Marks VJ (2004) The running bolster suture for full-thickness skin grafts. Dermatol Surg 30(1): 92–4
2. Blair VP, Brown JB (1968) The use and uses of large split skin grafts of intermediate thickness. Plast Reconstr Surg 42(1): 65–75
3. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J (2005) The global burden of diabetic foot disease. Lancet 366(9498): 1719–24
4. Brown JE, Holloway SL (2018) An evidence-based review of split-thickness skin graft donor site dressings. Int Wound J 15(6): 1000–9
5. Grande DJ, Elson DM (2021) Skin Grafting - Indications. Medscape. Available at: https://emedicine.medscape.com/ article/1129479-overview#a8 (accessed 11.08.21)
6. Ha TS, Cho YS, Kim DH et al (2007) The clinical effectiveness of medifoam-silver in burns. Journal of the Korean Burn Society 10(1): 65–70
7. Joyce KM, Joyce CW, Mahon N, Kelly JL (2015) Use of a barbed suture tie-over technique for skin graft dressings: a case series. Arch Plast Surg 42(3): 341–5
8. Kromka W, Cameron M, Fathi R (2018) Tie-over bolster dressings vs basting sutures for the closure of full-thickness skin grafts: a review of the literature. J Cutan Med Surg 22(6): 602–6
9. Lee SM, Park IK, Kim YS et al (2016a) Superior absorption and retention properties of foam-film silver dressing versus other commercially available silver dressing. Biomater Res 20: 22
10. Lee SM, Park IK, Kim YS et al (2016b) Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater Res 20: 15
11. Margolis DJ, Kantor J, Berlin JA (1999) Healing of diabetic neuropathic foot ulcers receiving standard treatment. A metaanalusis. Diabetes Care 22(5): 692–5
12. Mccartan B, Dinh T (2012) The use of split-thickness skin grafts on diabetic foot ulcerations: a literature review. Plast Surg Int 2012: 715273.
13. Ogawa R, Hyakusoku H, Ono S (2007) Useful tips for successful skin grafting. J Nippon Med Sch 74(6): 386–92
14. Pak CS, Park DH, Oh TS et al (2019) Comparison of the efficacy and safety of povidone-iodine foam dressing (Betafoam), hydrocellular foam dressing (Allevyn), and petrolatum gauze for split-thickness skin graft donor site dressing. Int Wound J 16(2): 379–86
15. Park YO, Minn KW, Hur JP (2002) The effect of Medifoam (hydrophilic polyurethane foam) dressing in split thickness skin graft donor site. Journal of the Korean Society of Plastic and Reconstructive Surgeons 29(4): 297–301
16. Ramanujam CL, Stapleton JJ, Kilpadi KL et al (2010) Splitthickness skin grafts for closure of diabetic foot and ankle wounds: a retrospective review of 83 patients. Foot Ankle Spec 3(5): 231–40
17. Sakurai A, Hashikawa K, Yokoo S et al (2007) Simple dressing technique using polyurethane foam for fixation of skin grafts. Dermatol Surg 33(8) 976–9
18. SMARTPORE Technology® Made Easy (2018) Wounds International Available from: https://www. woundsinternational.com/resources/details/smartpore-madeeasy (accessed 21.06.22)
19. Steele L, Brown A, Xie F (2020) Full-thickness skin graft fixation techniques: a review of the literature. J Cutan Aesthet Surg 13(3): 191–6
20. Stevens DL, Bisno AL, Chambers HF et al (2014) Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59(2): e10–52
21. Wiechula R (2003) The use of moist wound-healing dressings in the management of split-thickness skin graft donor sites: a systematic review. Int J Nurs Pract 9(2): S9–17
22. Yammine K, Assi C (2019) A meta-analysis of the outcomes of split-thickness skin graft on diabetic leg and foot ulcers. Int J Low Extrem Wounds 18(1): 23–30
This article is excerpted from the 《The Diabetic Foot Journal Vol 25 No 2 2022》 by Wound World.


