
伤口世界
电子邮件地址: 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。

- 星期五, 16 1月 2026
The Whitening, Moisturizing, Anti-aging Activities, and Skincare Evaluation of Selenium-Enriched Mung Bean Fermentation Broth




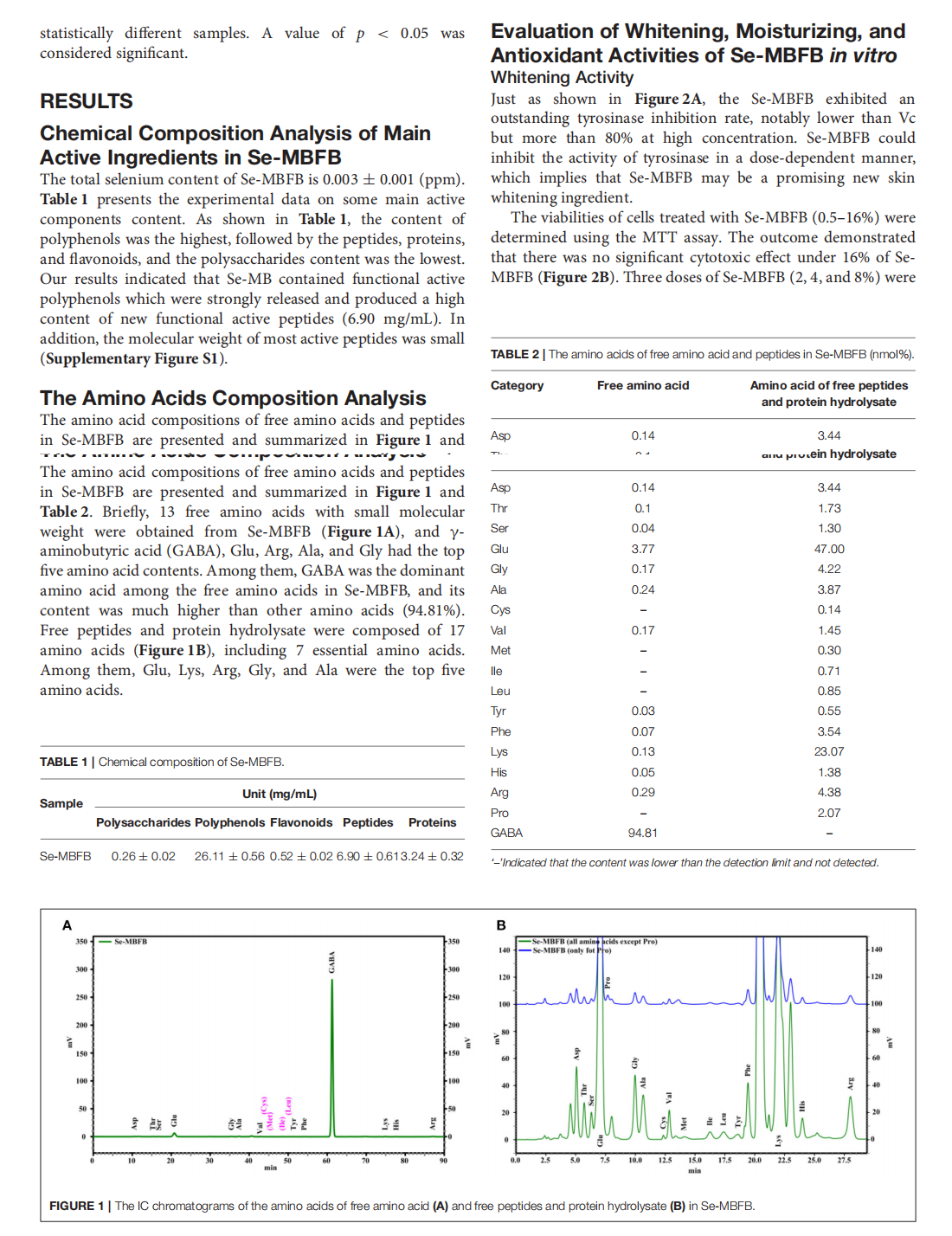
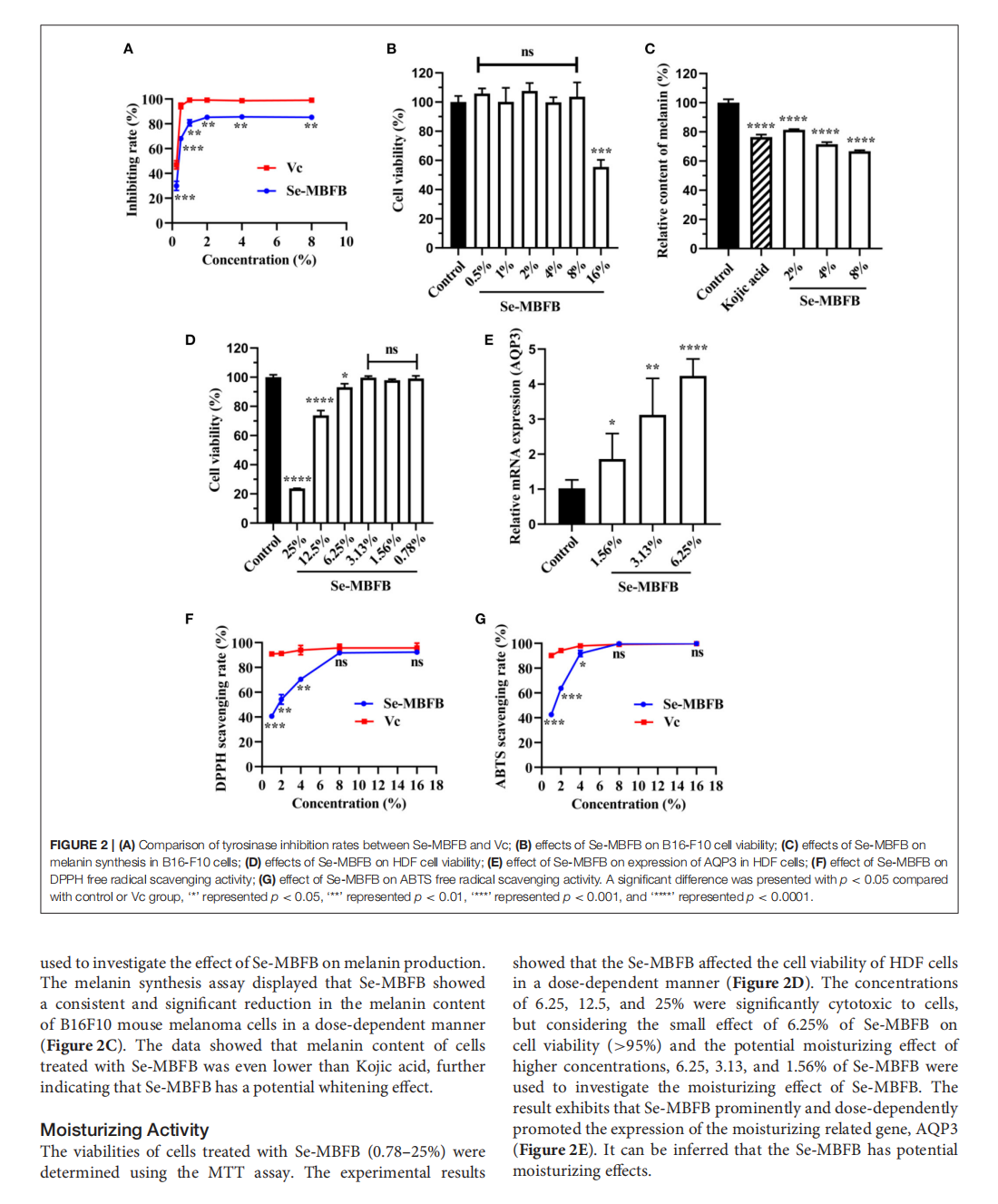

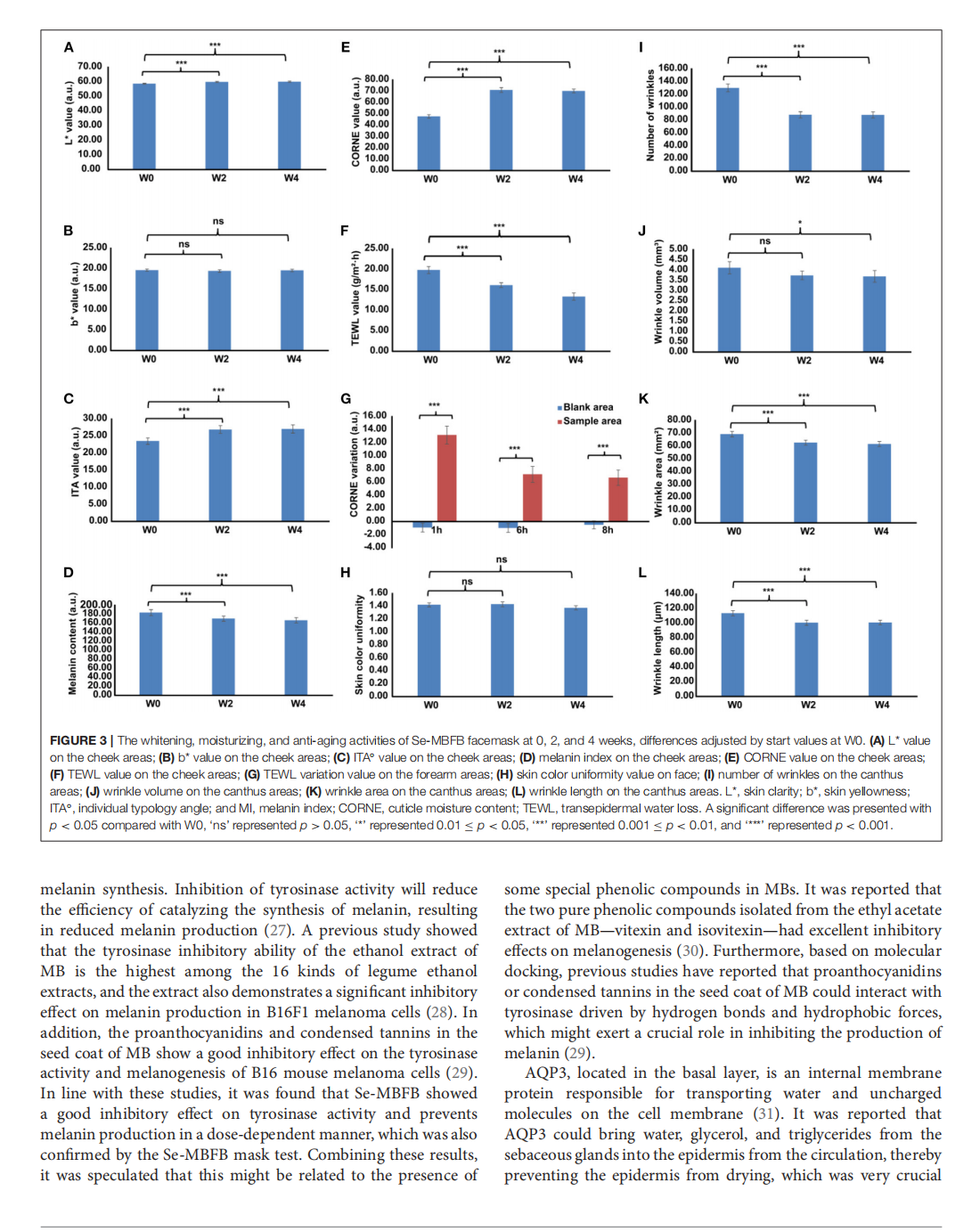
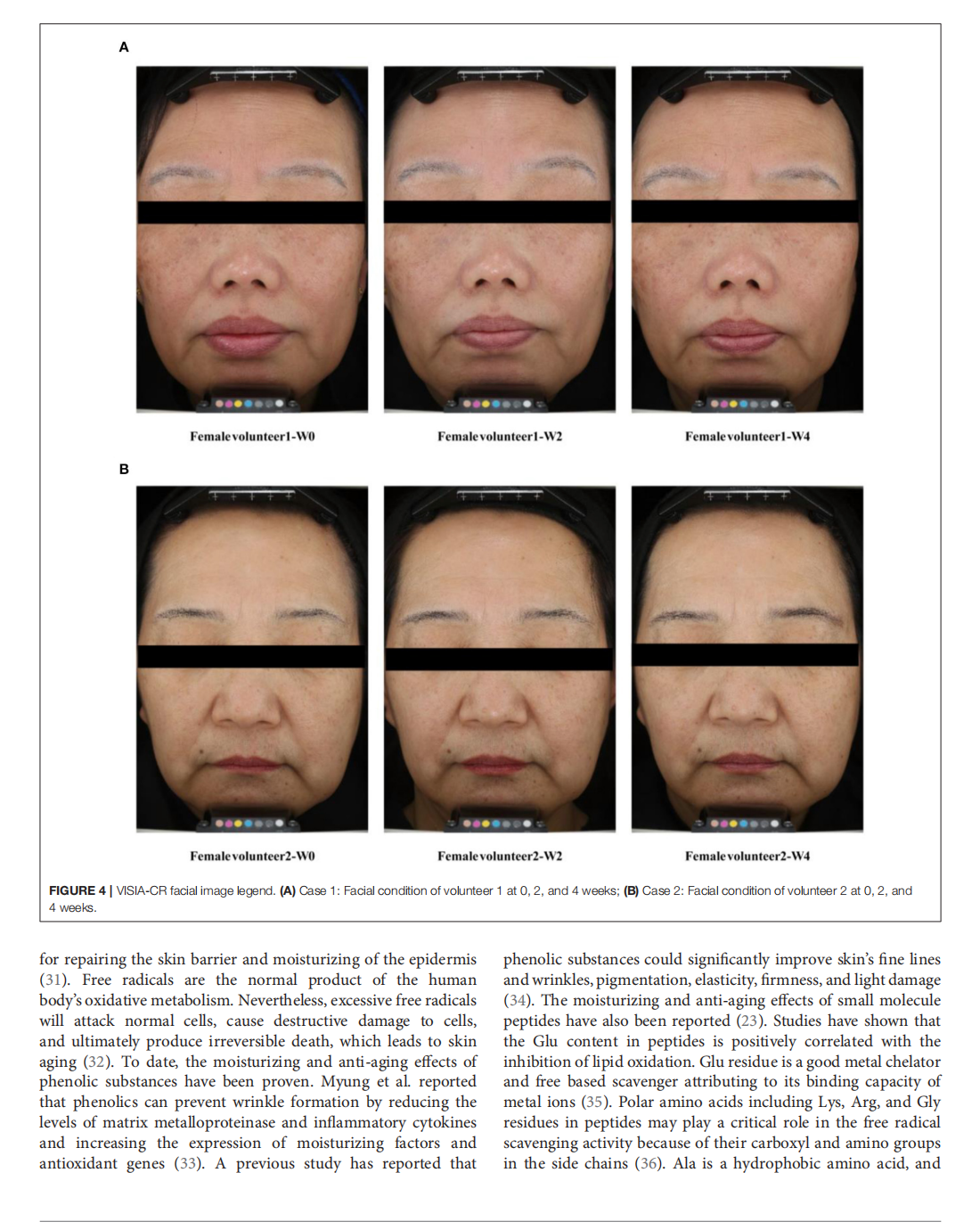


This article is excerpted from the《Frontiers in Nutrition》by Wound World

- 星期四, 15 1月 2026
Bridging clinic to home: domestic devices in dermatological diagnostics and treatments







This article is excerpted from the 《Frontiers in Digital Health》 by Wound World

- 星期三, 14 1月 2026
Knowledge, attitudes, and practices in adult patients and parents of pediatric atopic dermatitis patients: a cross-sectional study



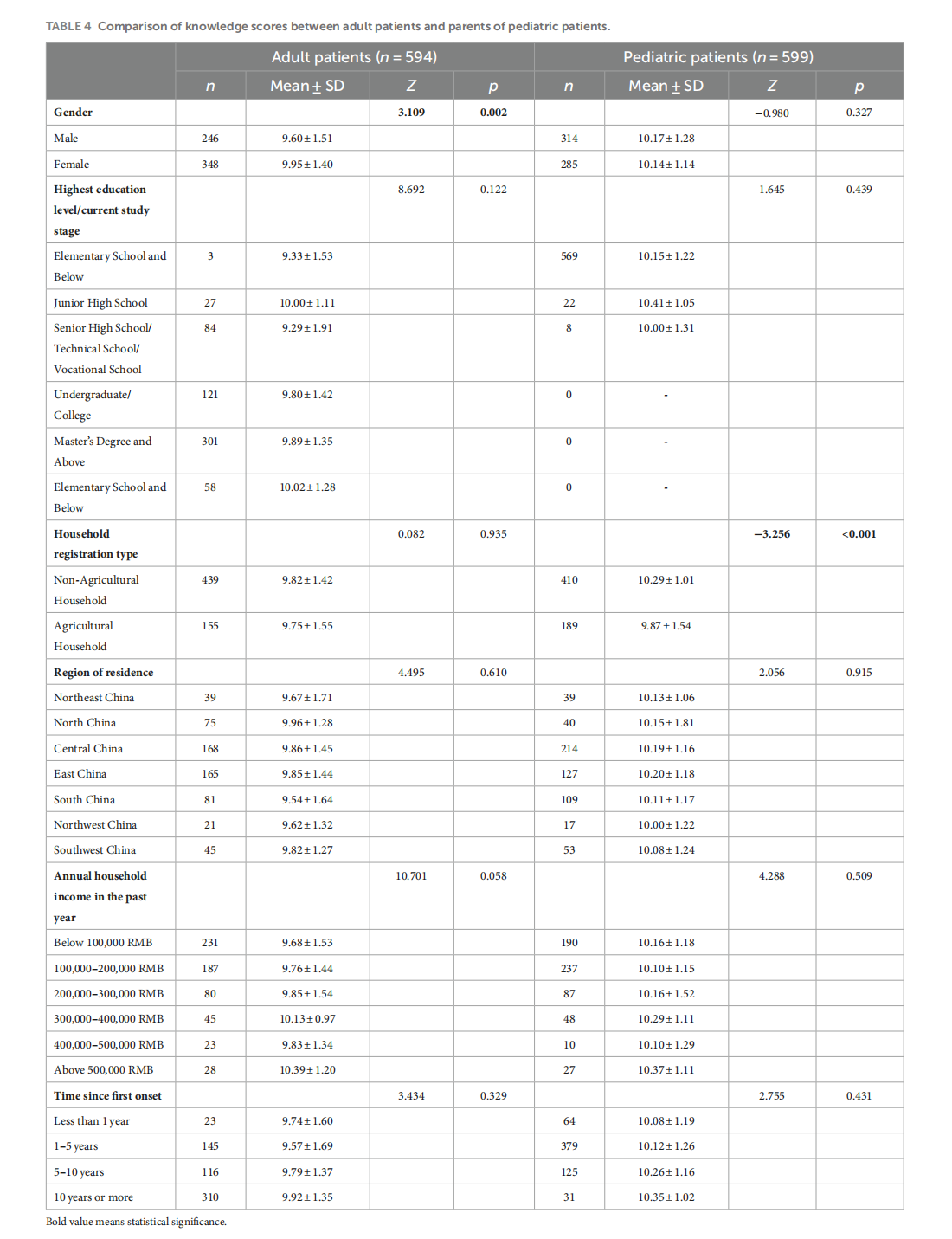
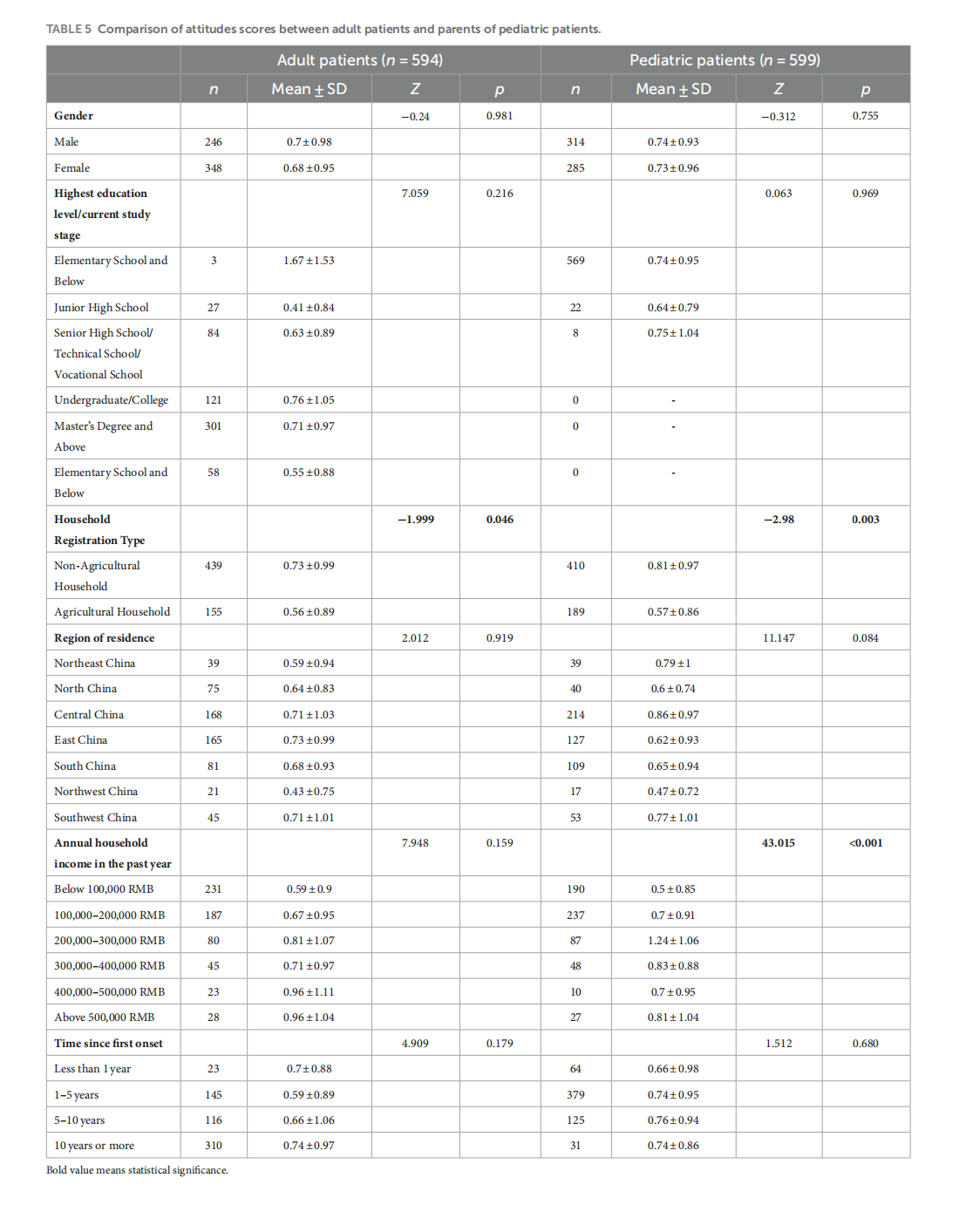
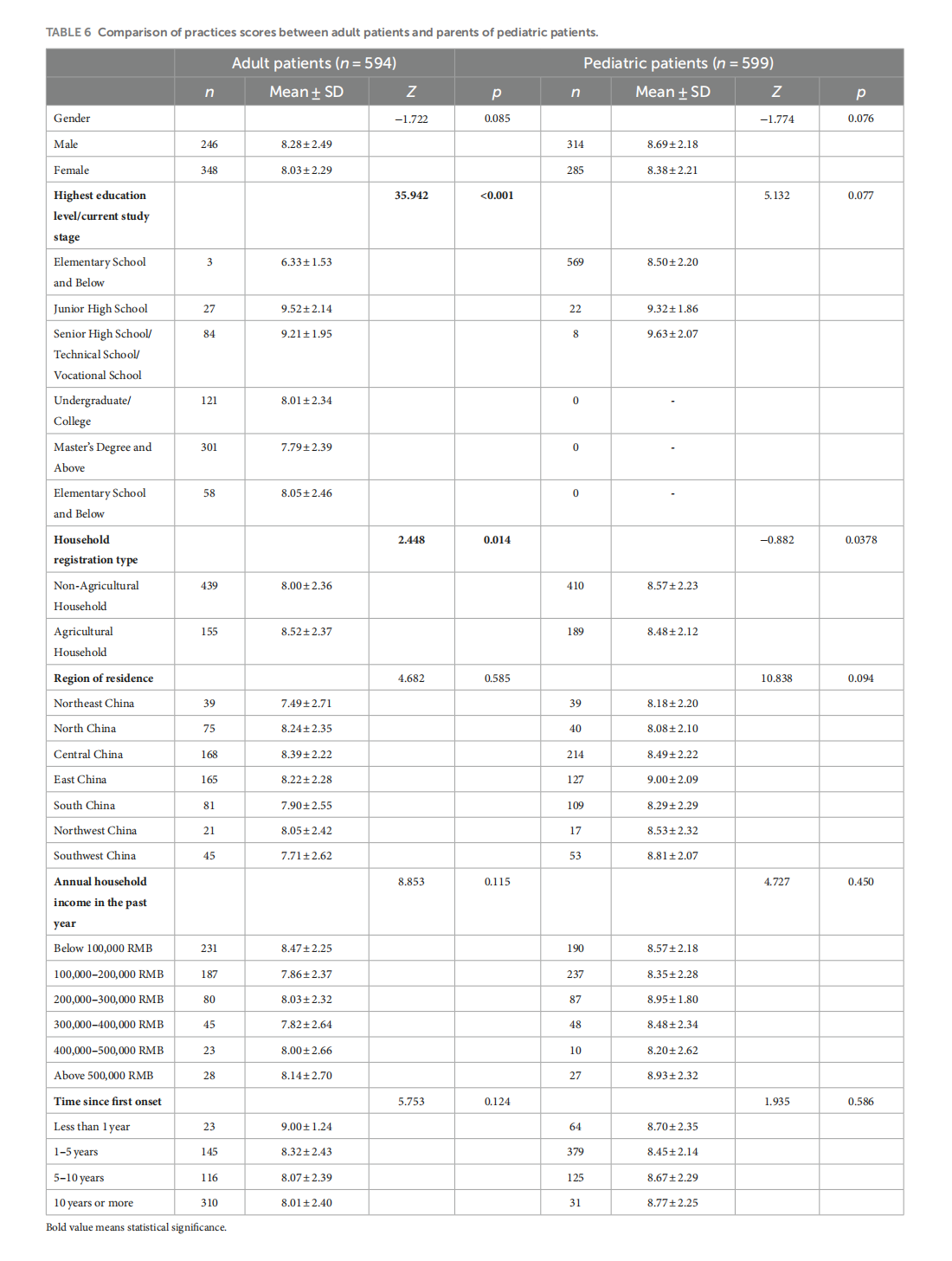
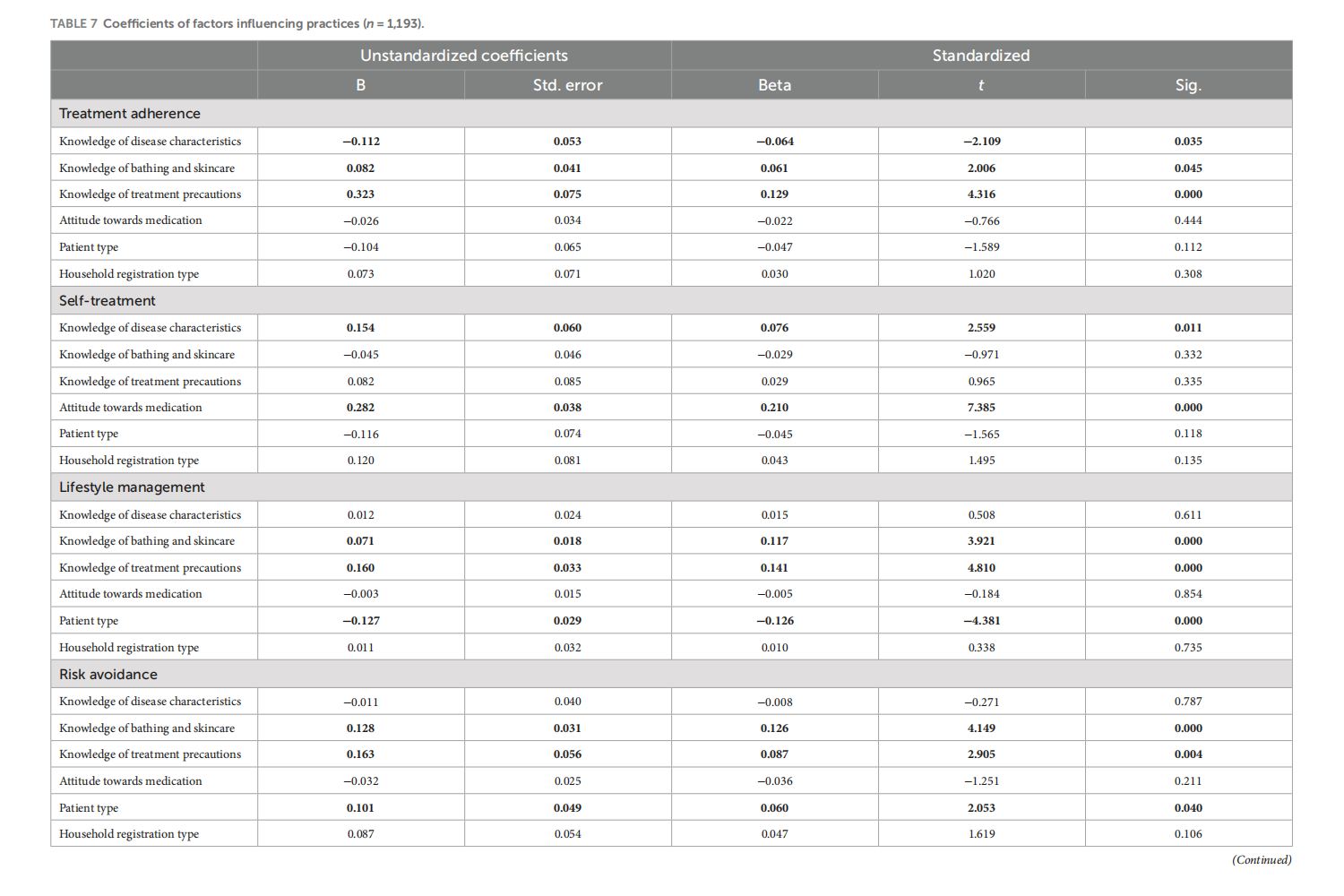
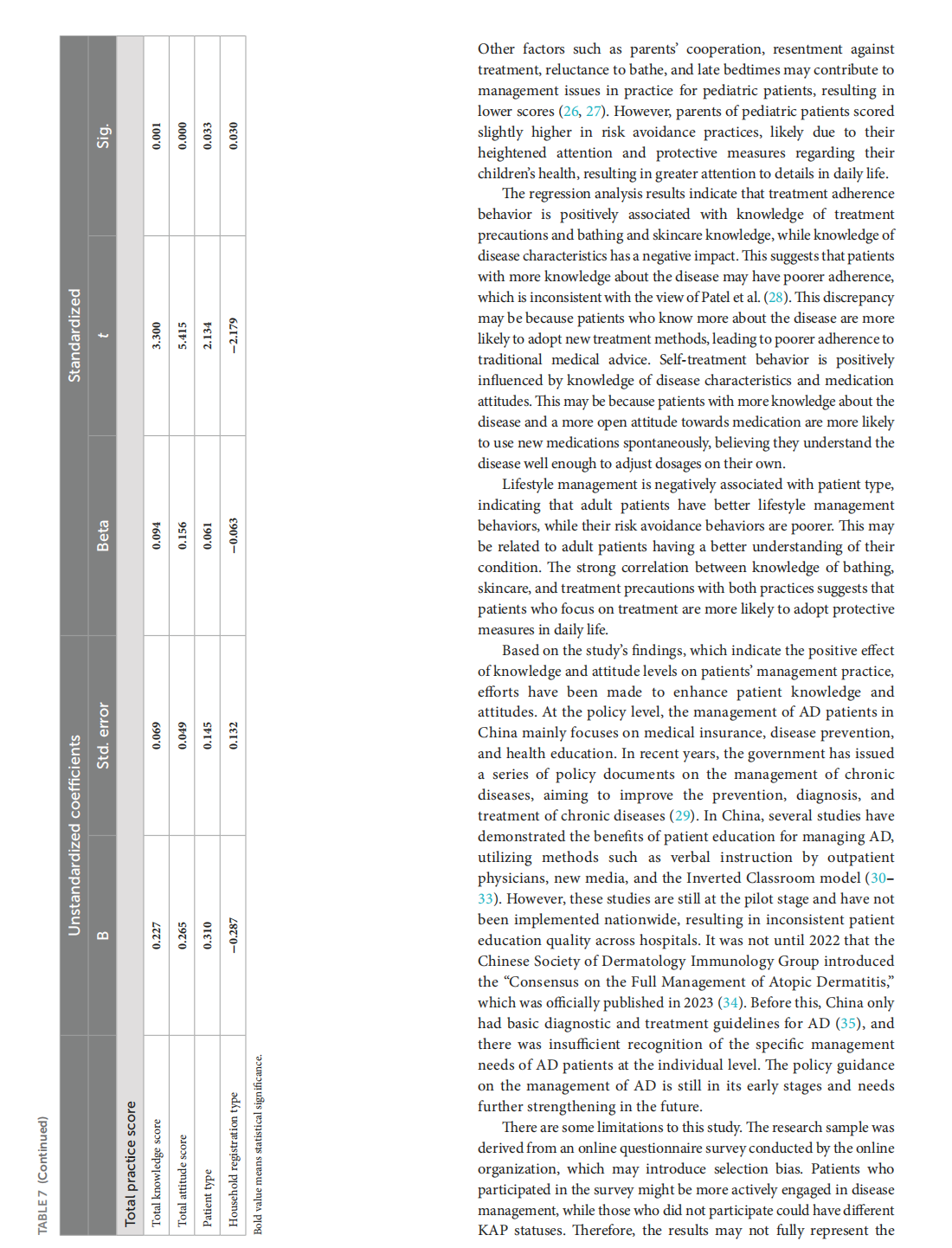


This article is excerpted from the《Frontiers in Public Health》 by Wound World

- 星期二, 13 1月 2026
Spectrum of sensitive skin in India: a collaborative expert position statement





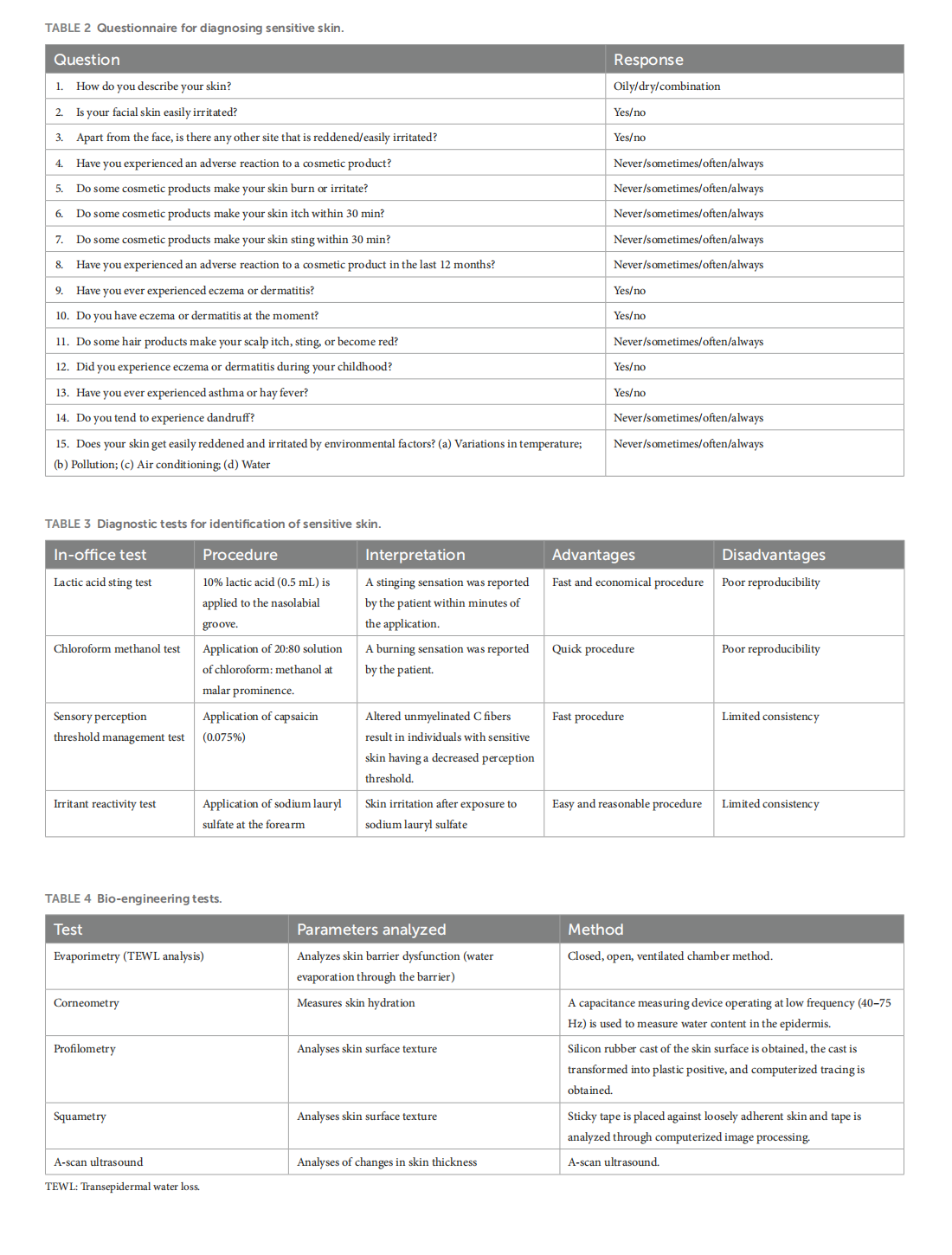






This article is excerpted from the 《Frontiers in Medicine》 by Wound World

- 星期一, 12 1月 2026
Medical Cannabis for the Treatment of Migraine in Adults: A Review of the Evidence


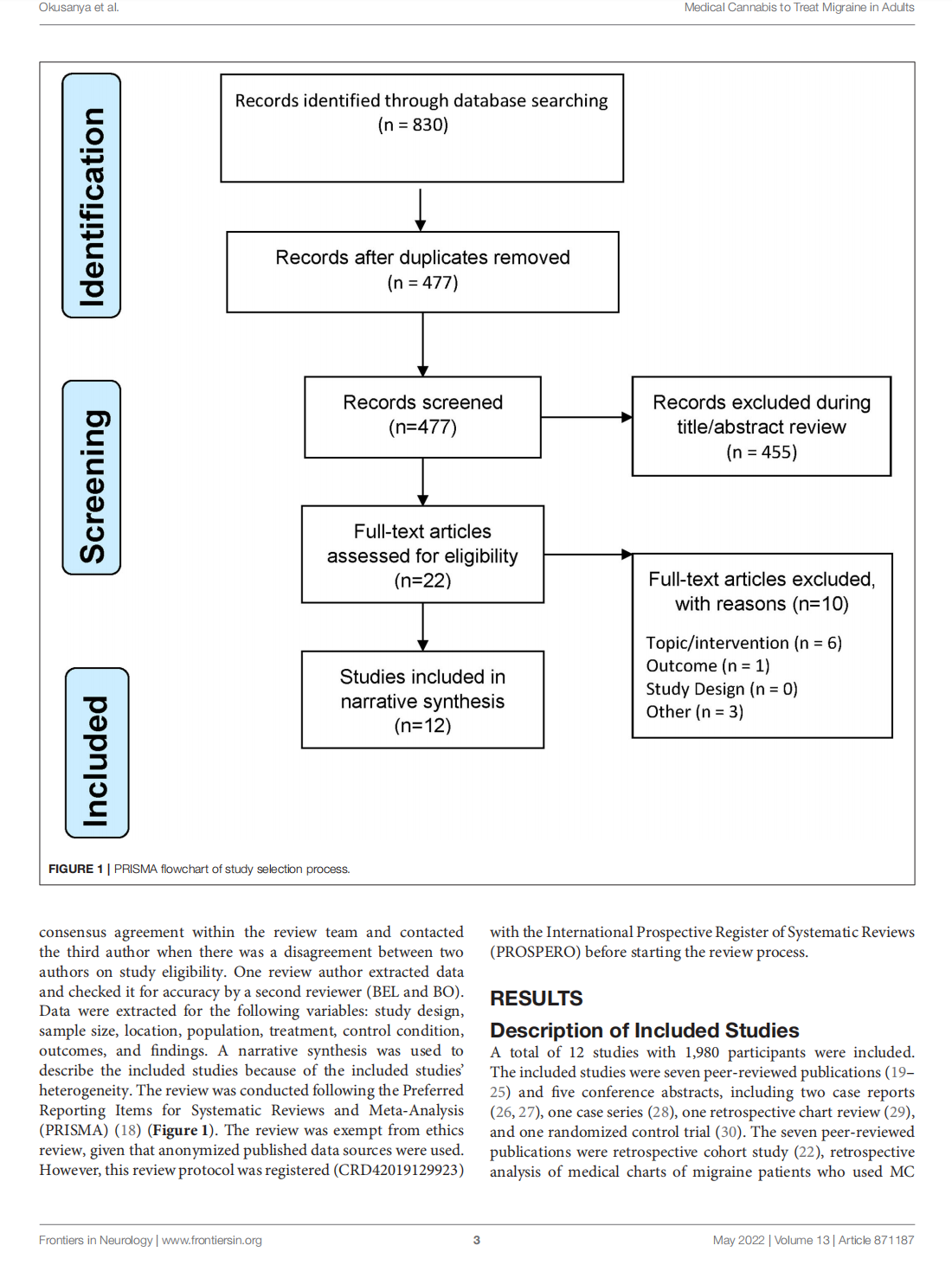
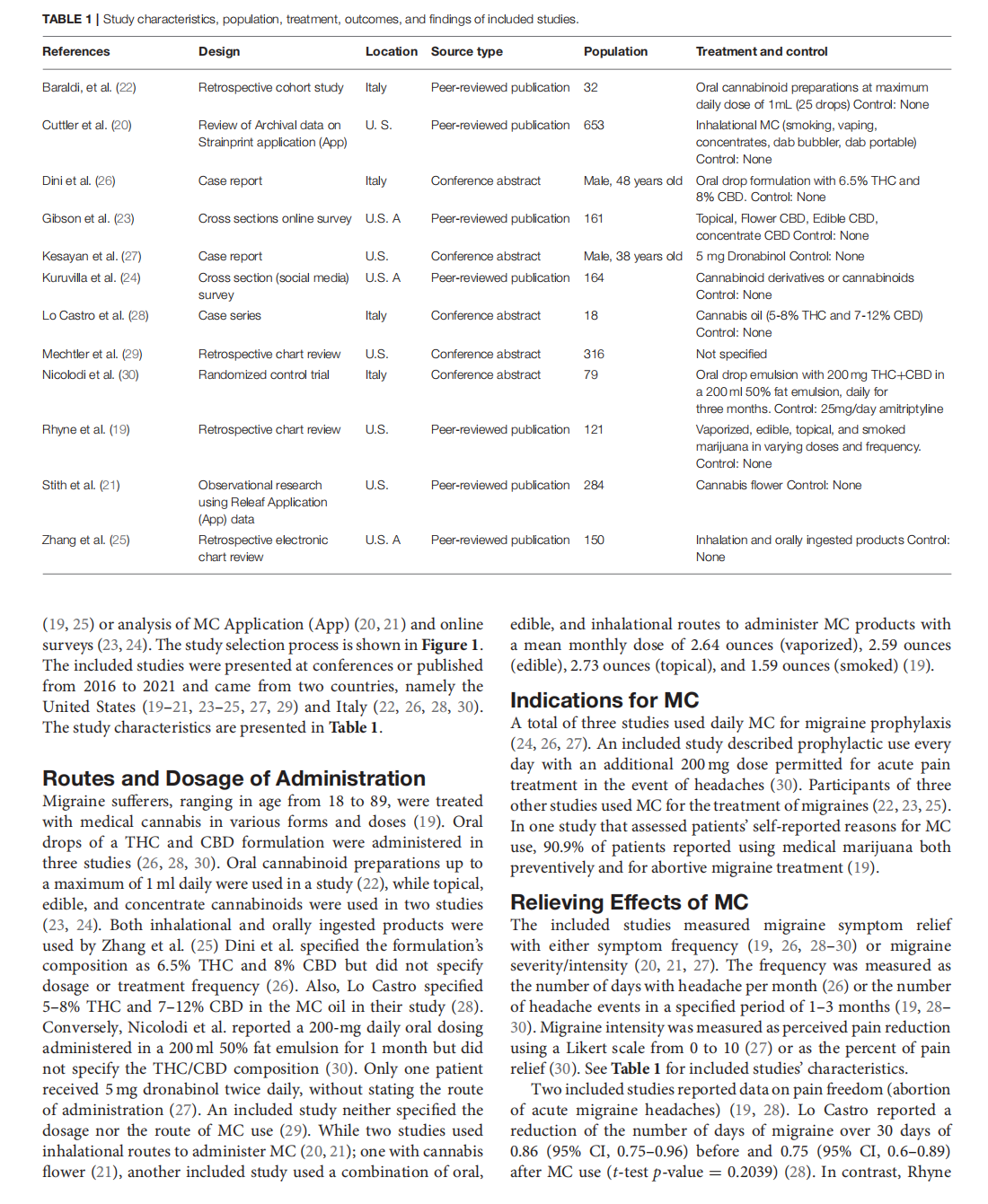



This article is excerpted from the《Frontiers in Neurology》by Wound World

- 星期五, 09 1月 2026
Integrative population pharmacokinetic/ pharmacodynamic analysis of nemonoxacin capsule in Chinese patients with community-acquired pneumonia




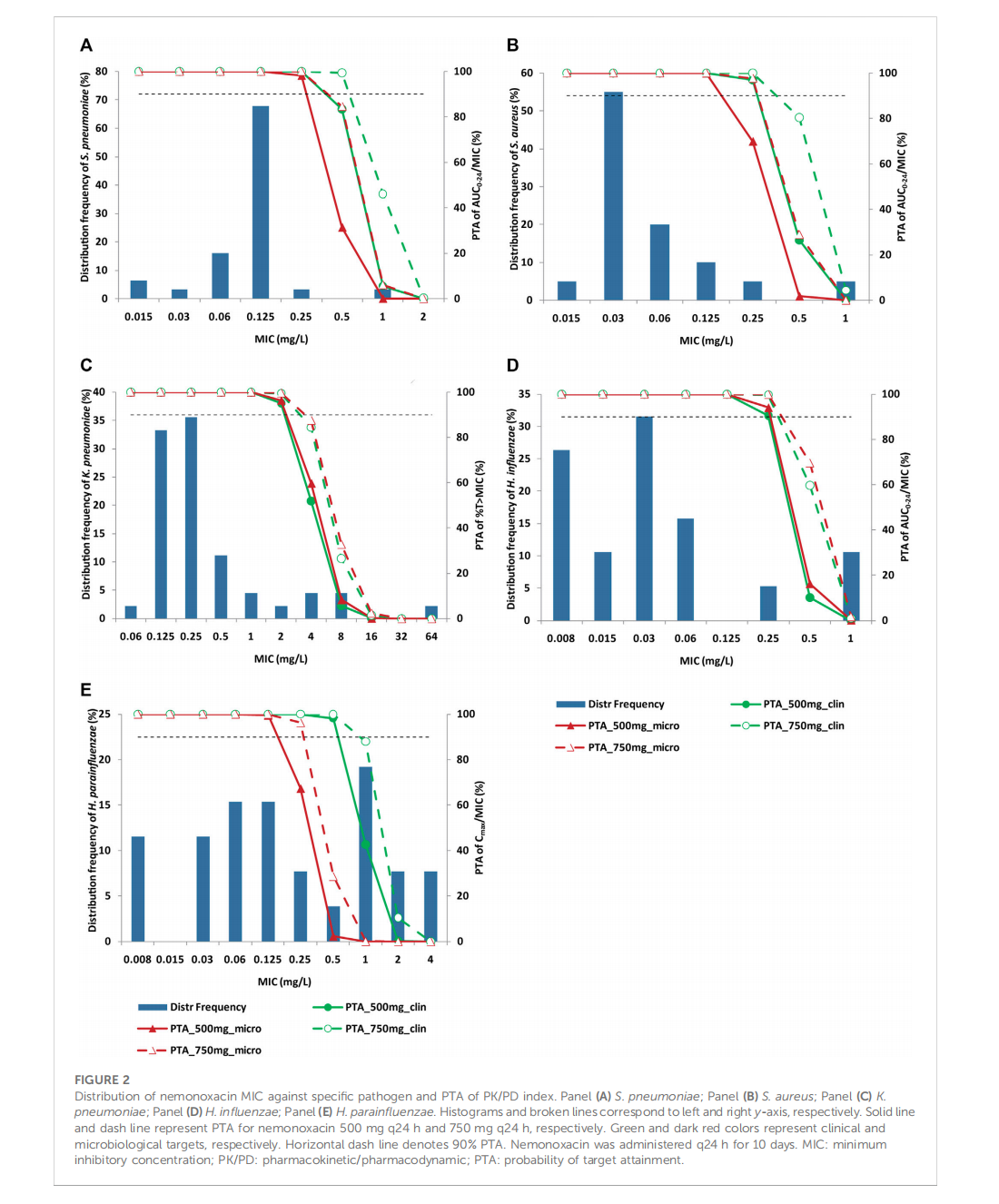
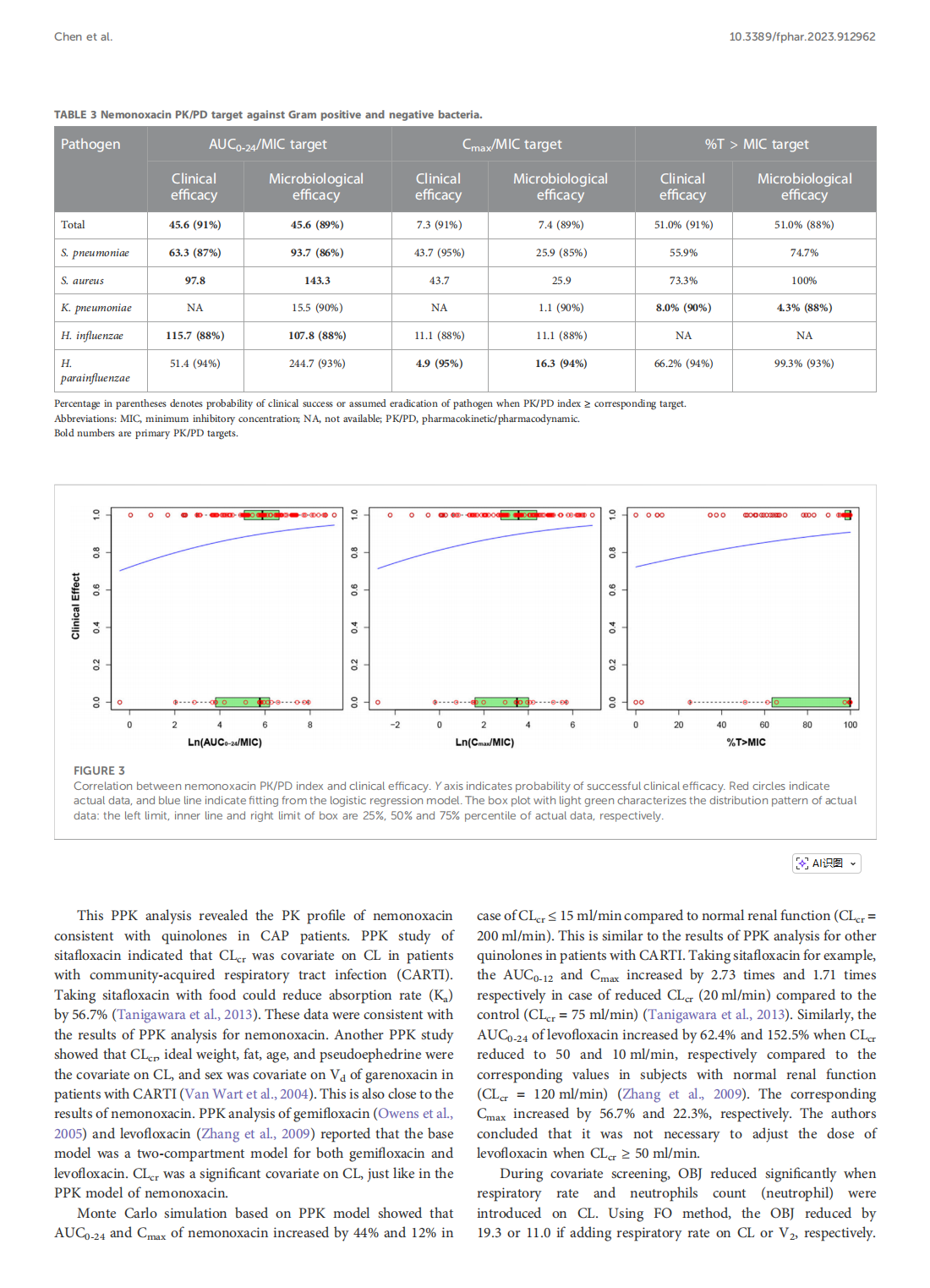
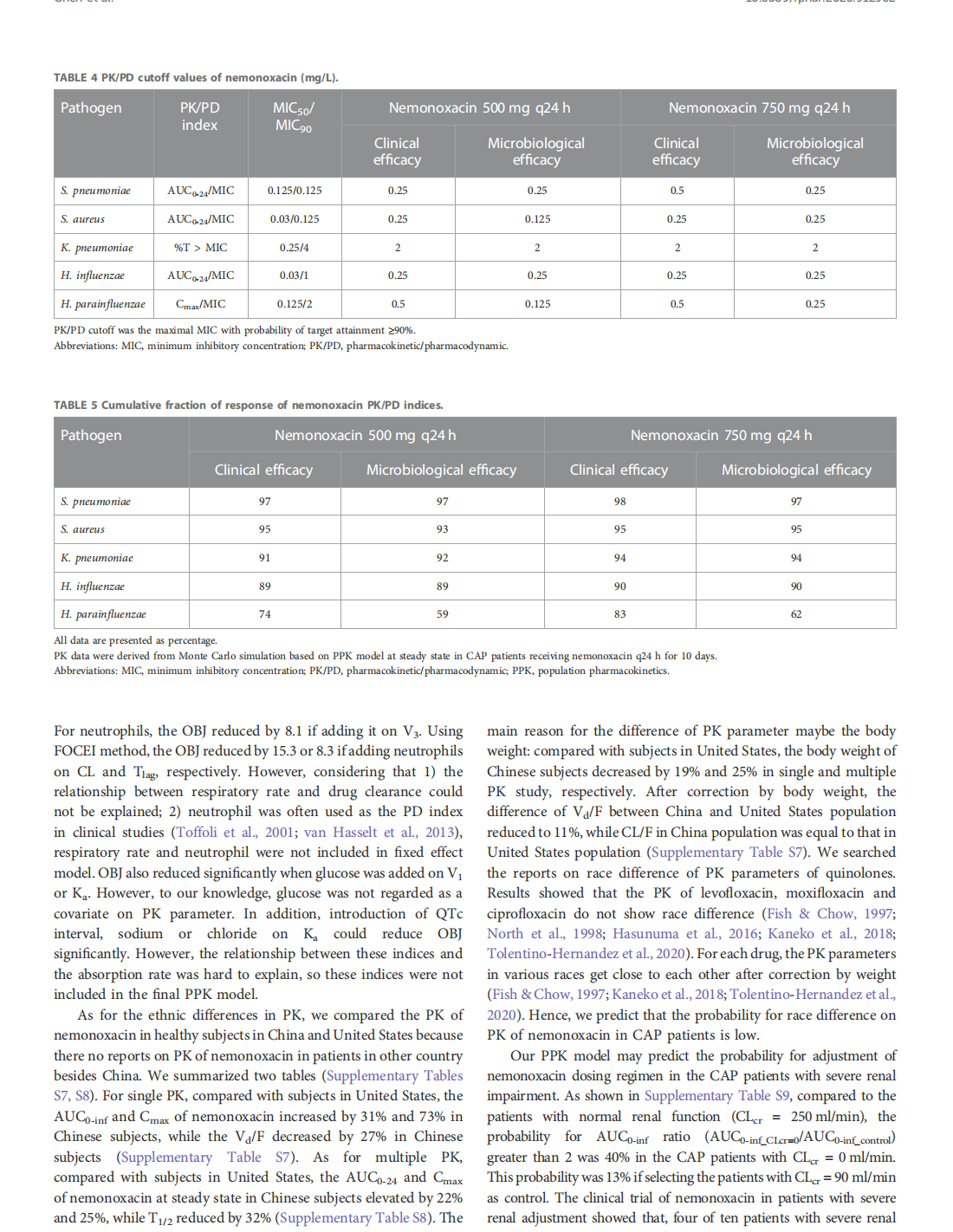




This article is excerpted from the《Frontiers in Pharmacology》by Wound World
