
伤口世界
电子邮件地址: 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。

- 星期一, 26 1月 2026
Implantable biomedical materials for treatment of bone infection



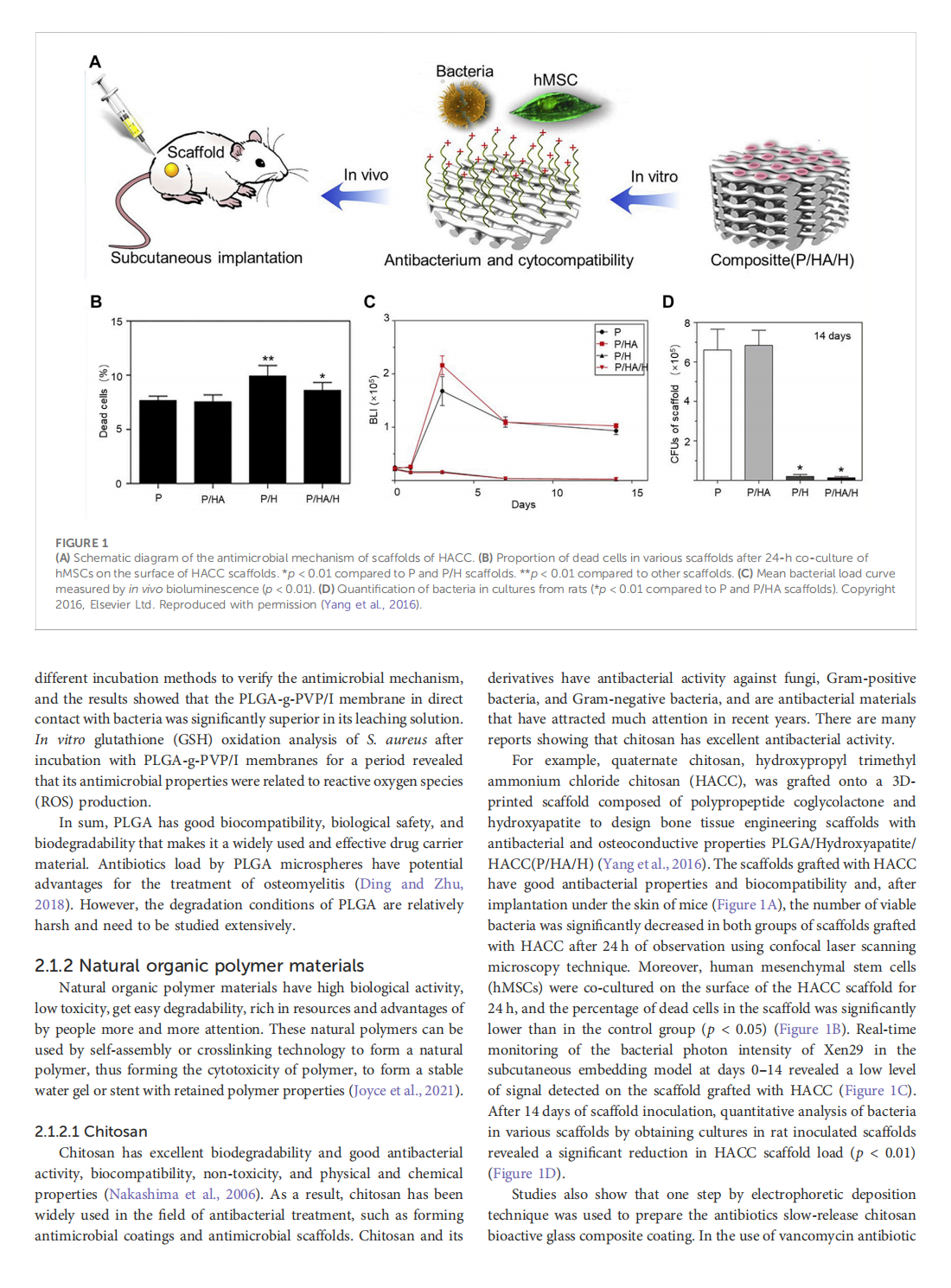


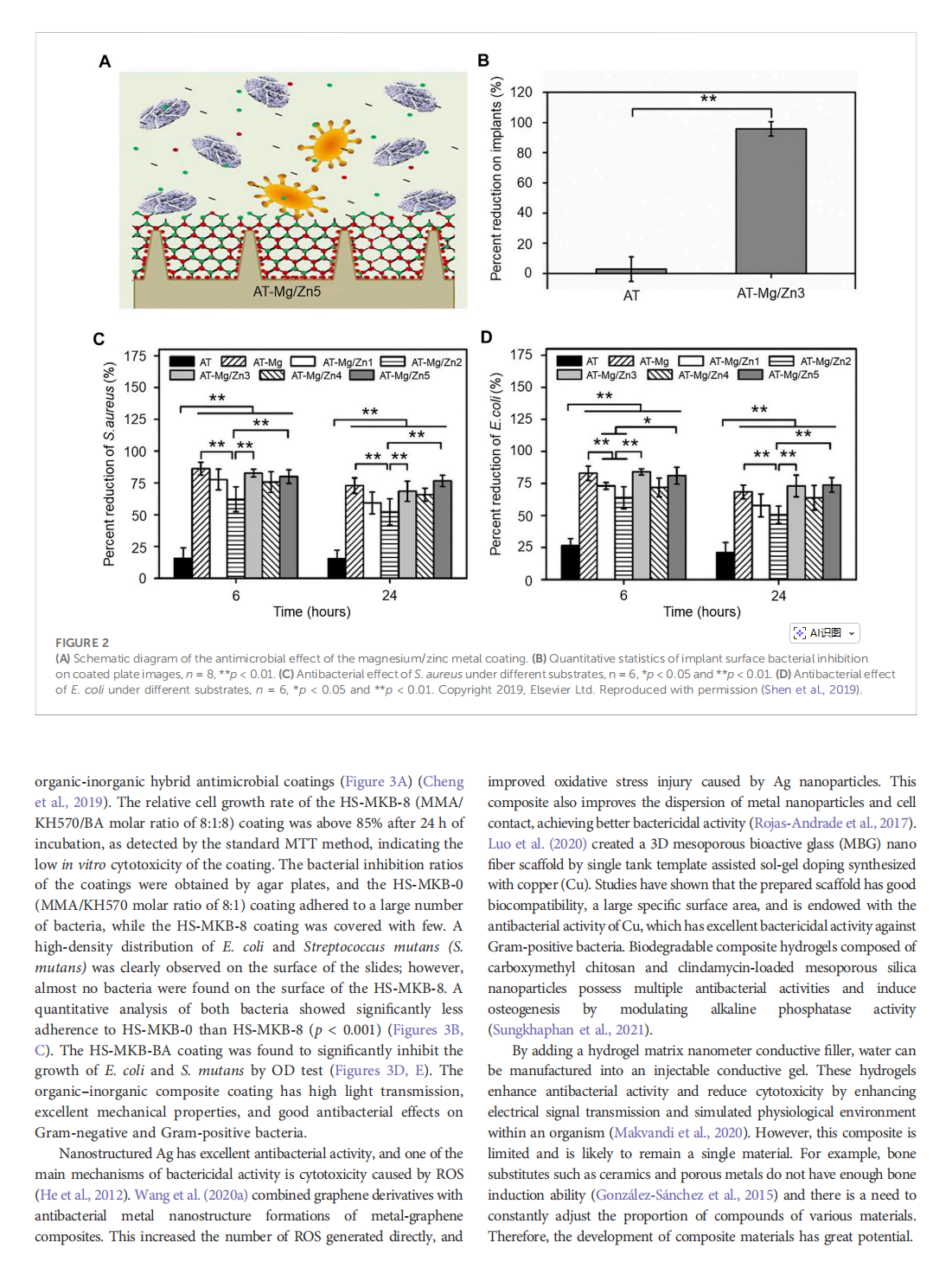

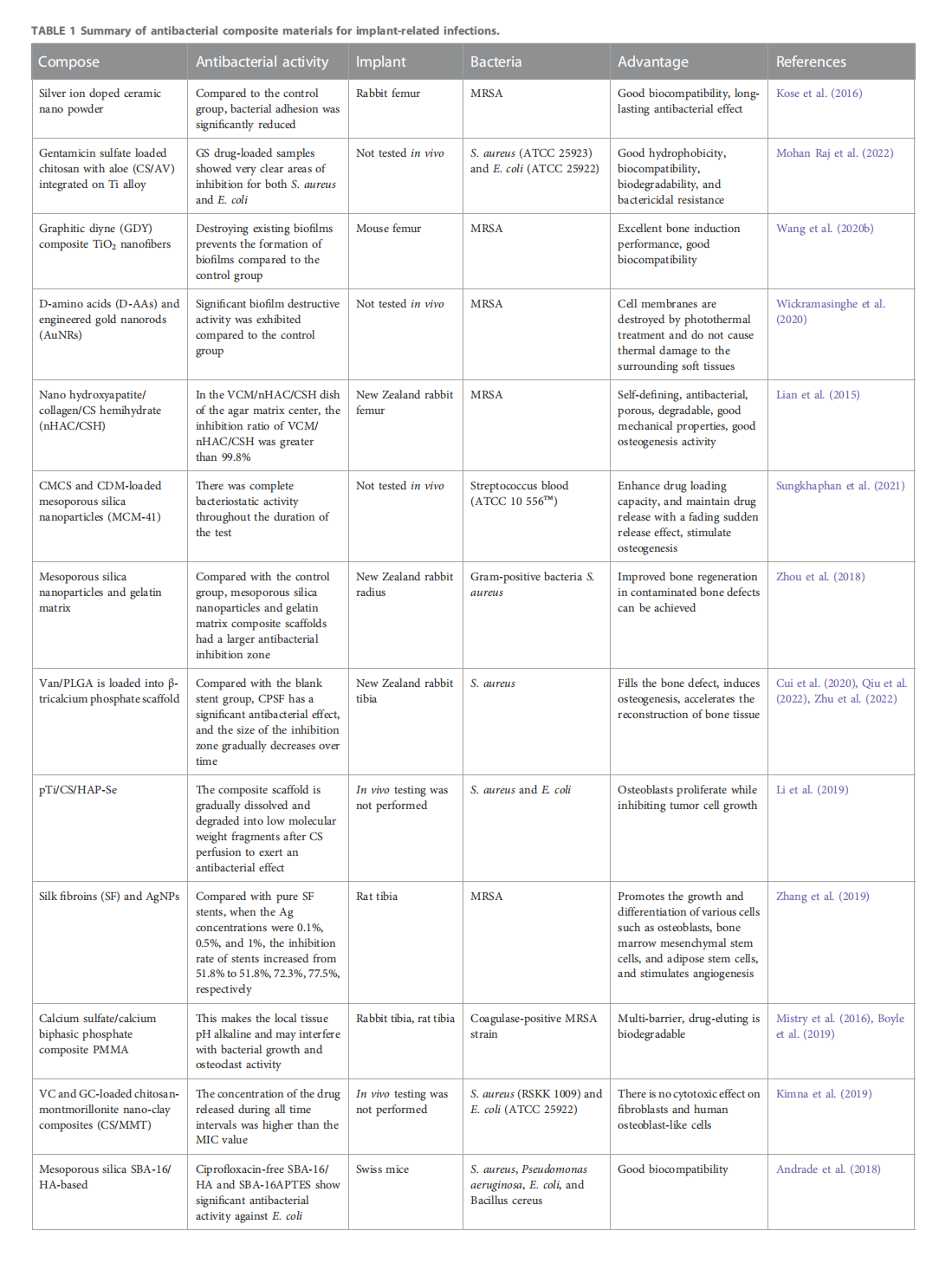
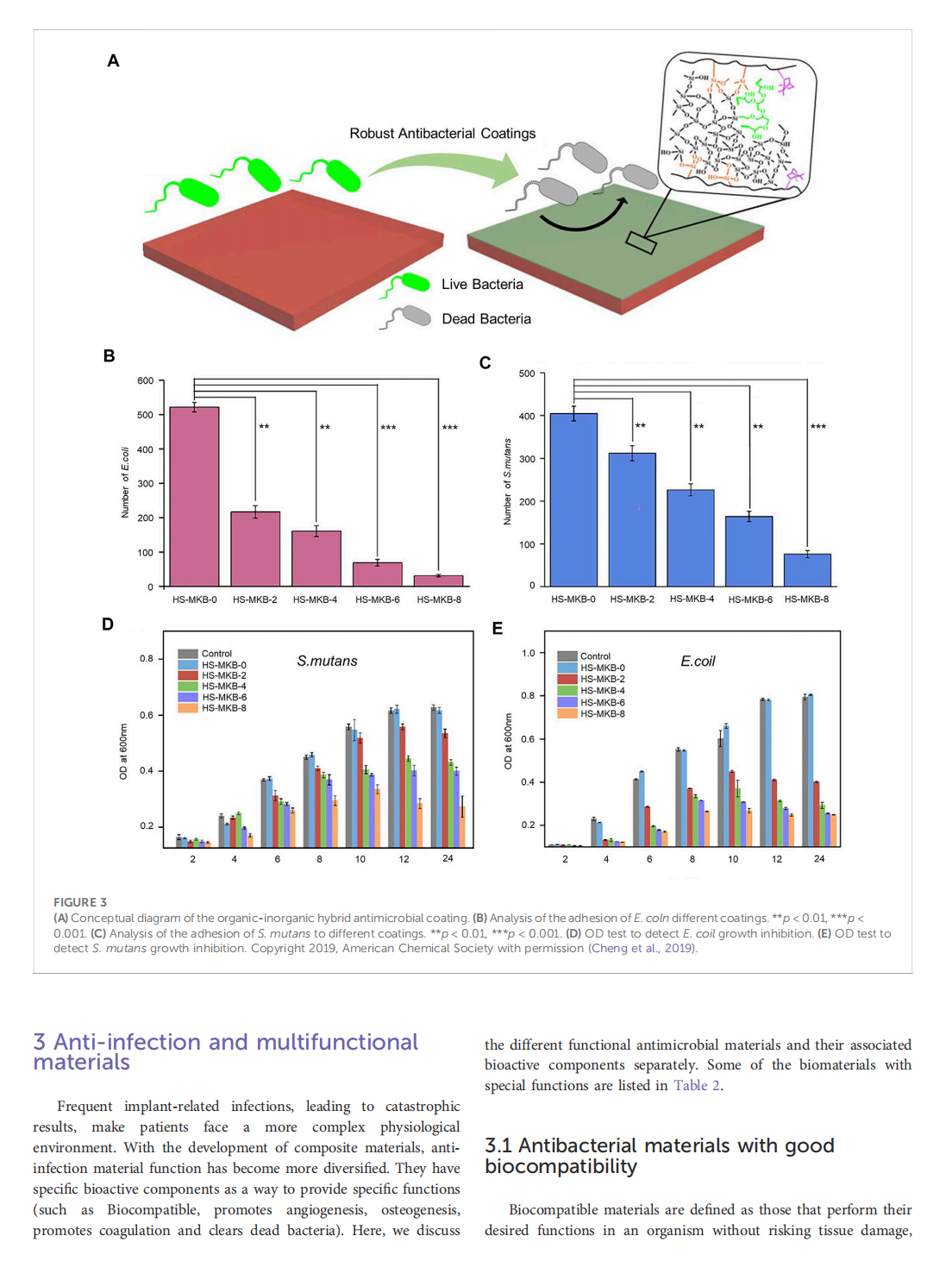
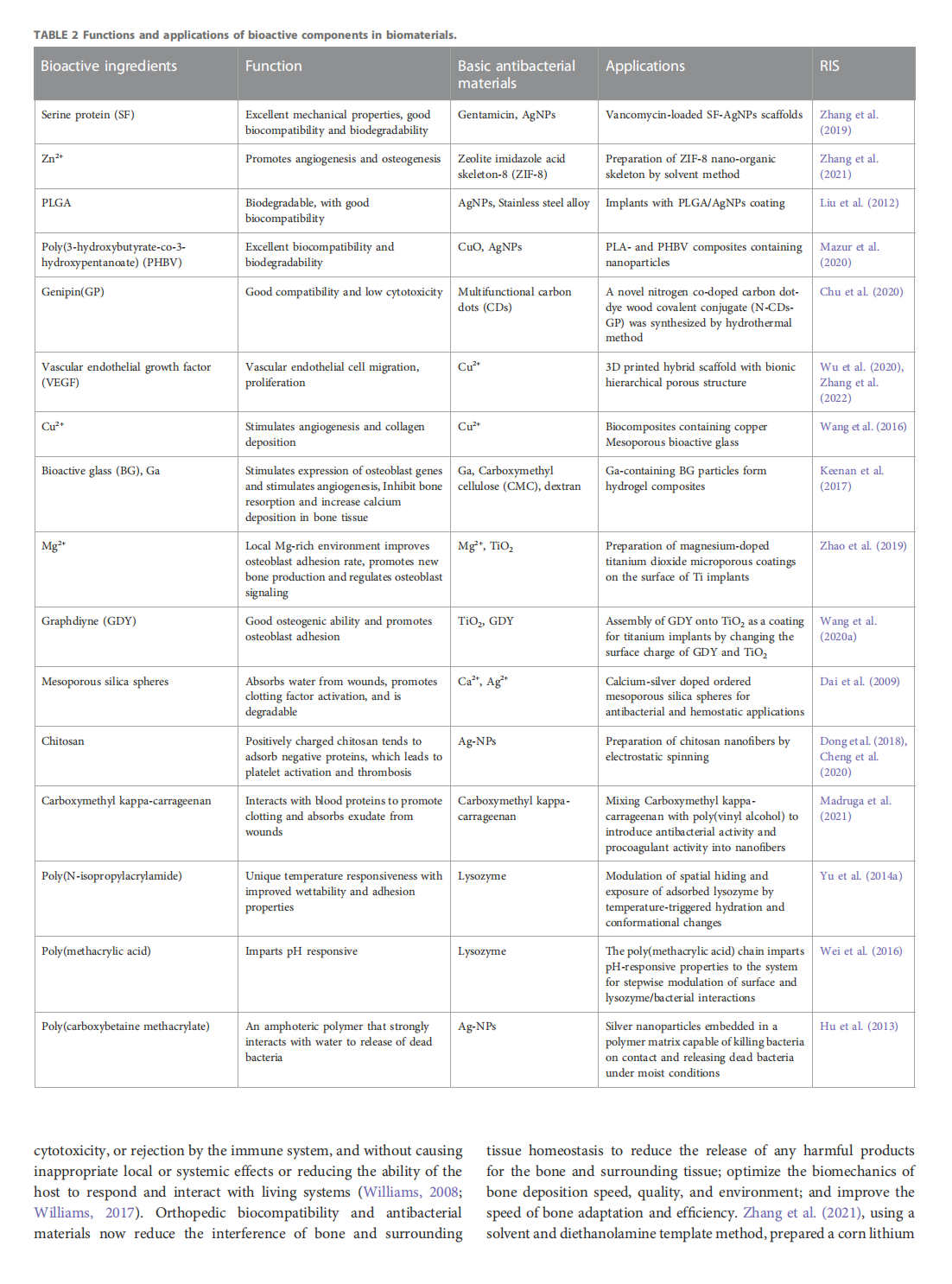

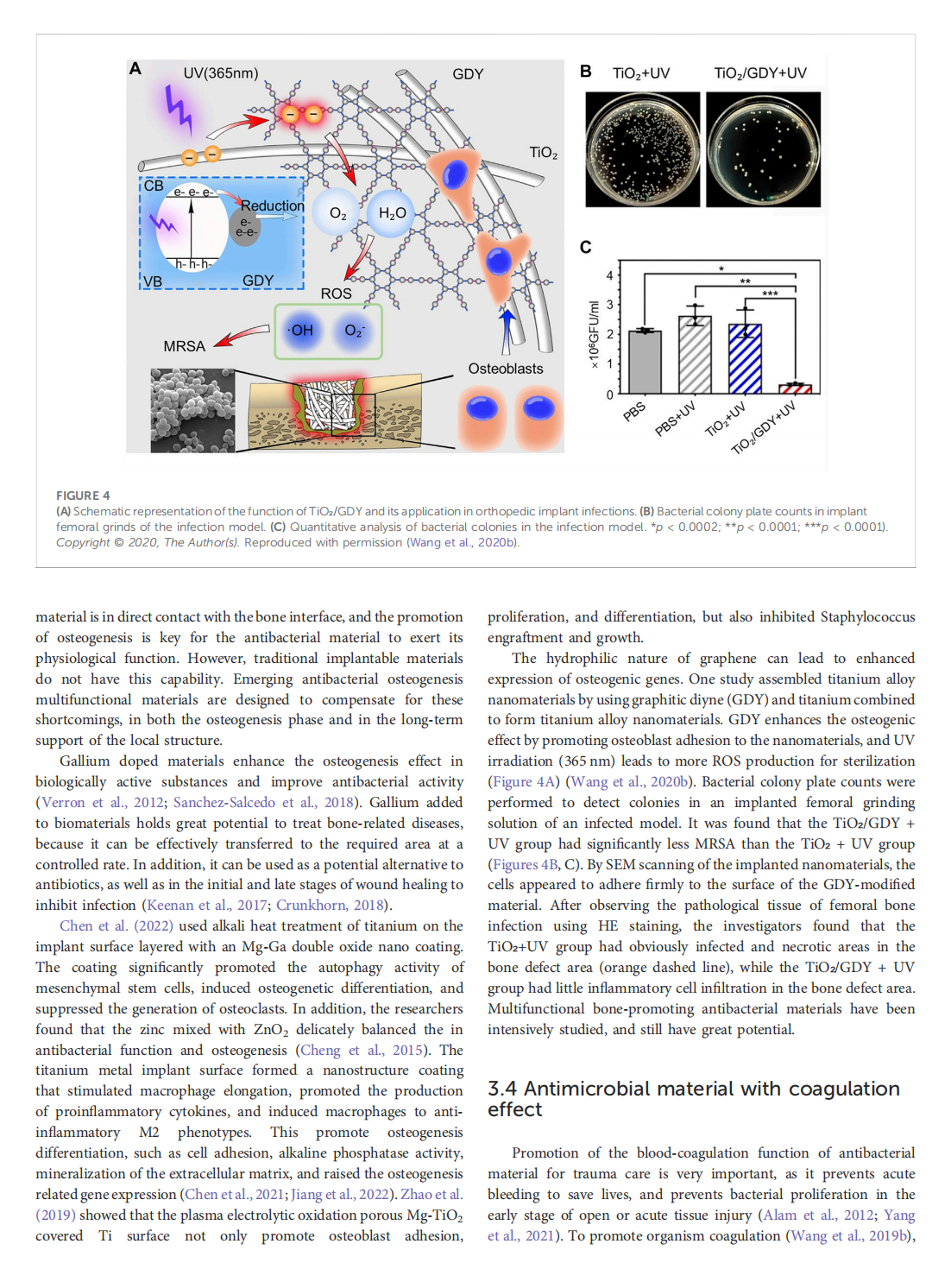

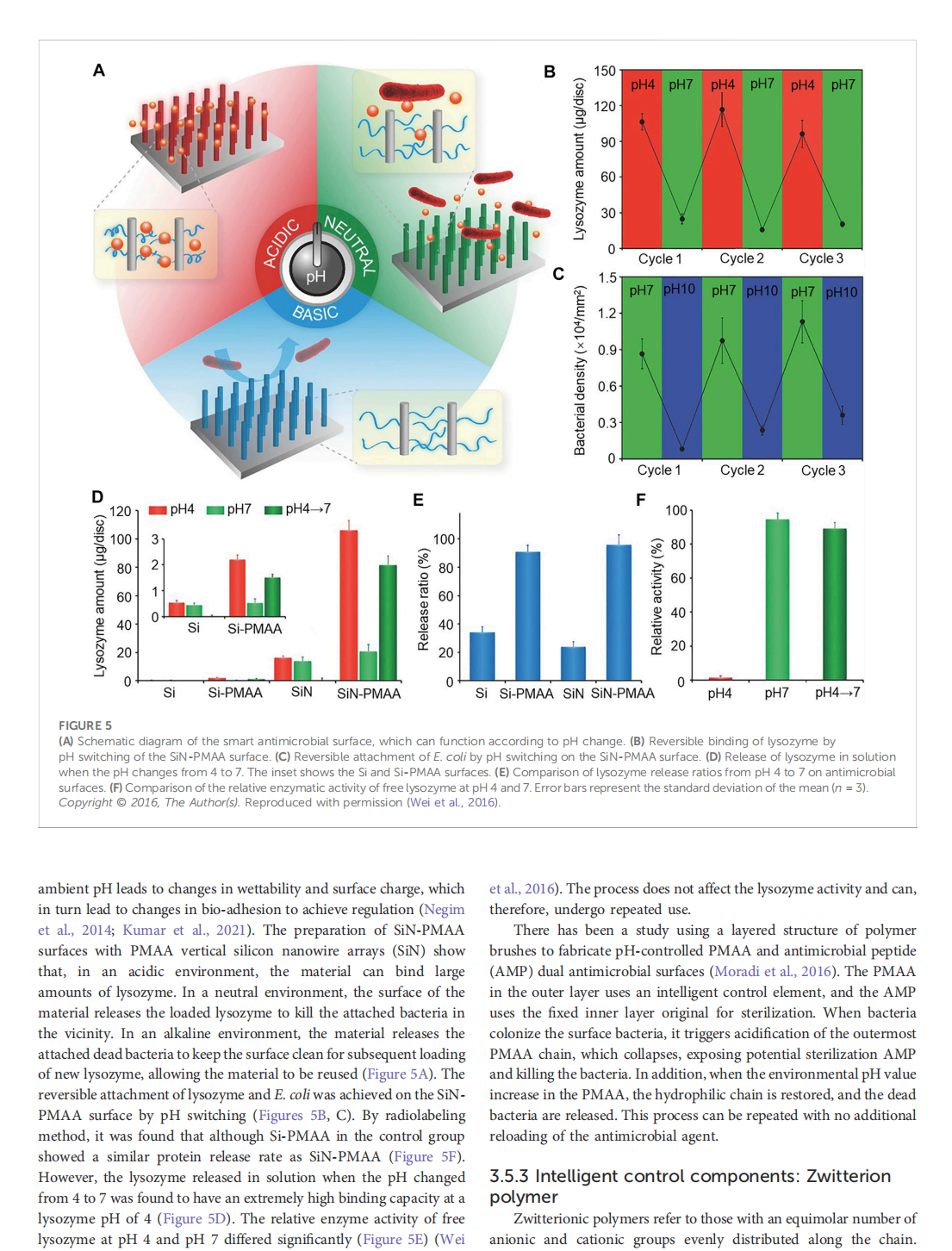





This article is excerpted from the《Frontiers in Bioengineering and Biotechnology》by Wound World

- 星期五, 23 1月 2026
Editorial: Best surgical treatment of breast cancer managed primarily with neoadjuvant medical therapy


This article is excerpted from the《Frontiers in Surgery》by Wound World

- 星期四, 22 1月 2026
Editorial: Going the Distance: Enabling 3D Cell Culture Systems for Biomedical Research and Drug Treatment


This article is excerpted from the 《Frontiers in Molecular Biosciences》 by Wound World

- 星期一, 19 1月 2026
Regenerative topical skincare: stem cells and exosomes


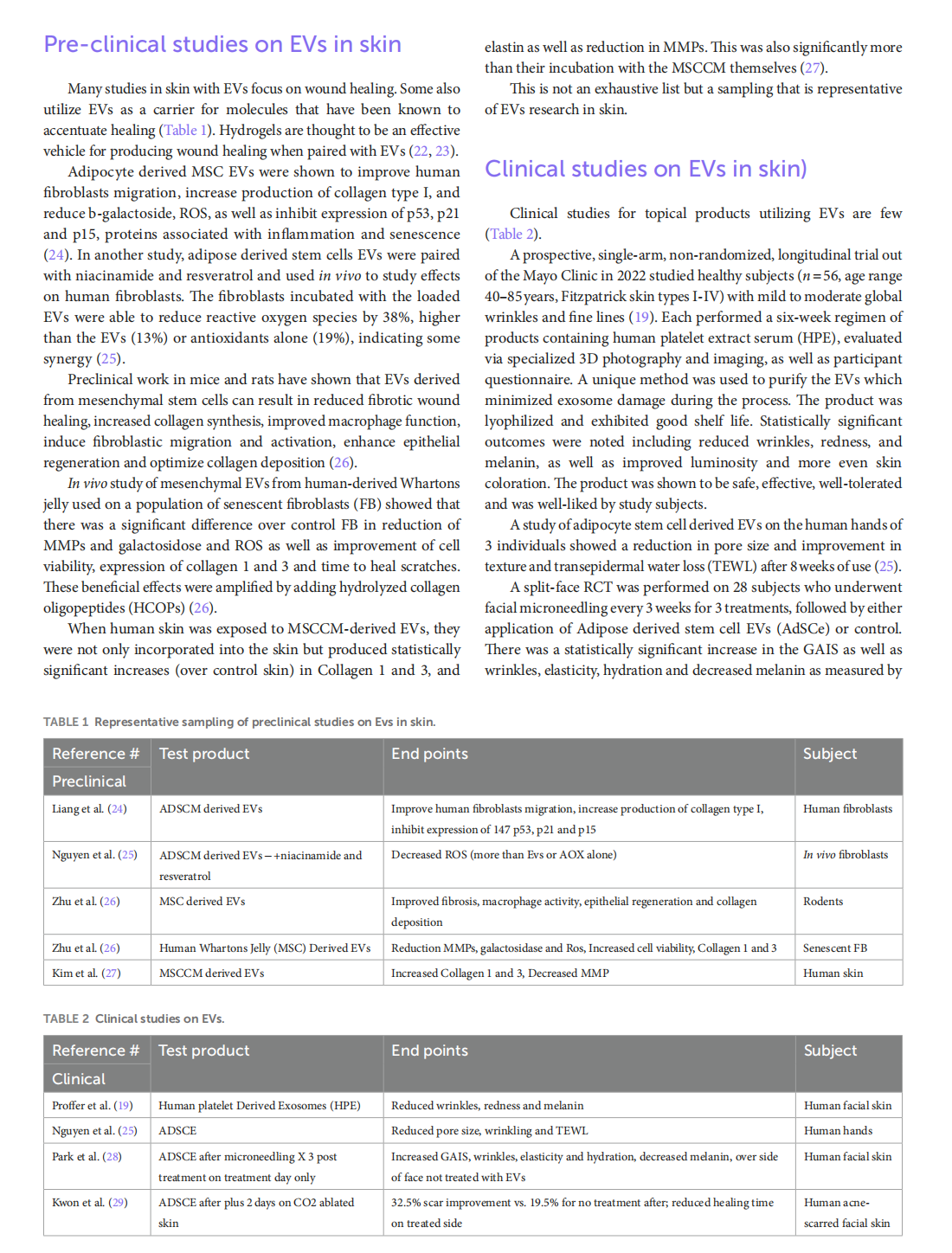


This article is excerpted from the 《Frontiers in Medicine》by Wound World

- 星期五, 16 1月 2026
The Whitening, Moisturizing, Anti-aging Activities, and Skincare Evaluation of Selenium-Enriched Mung Bean Fermentation Broth




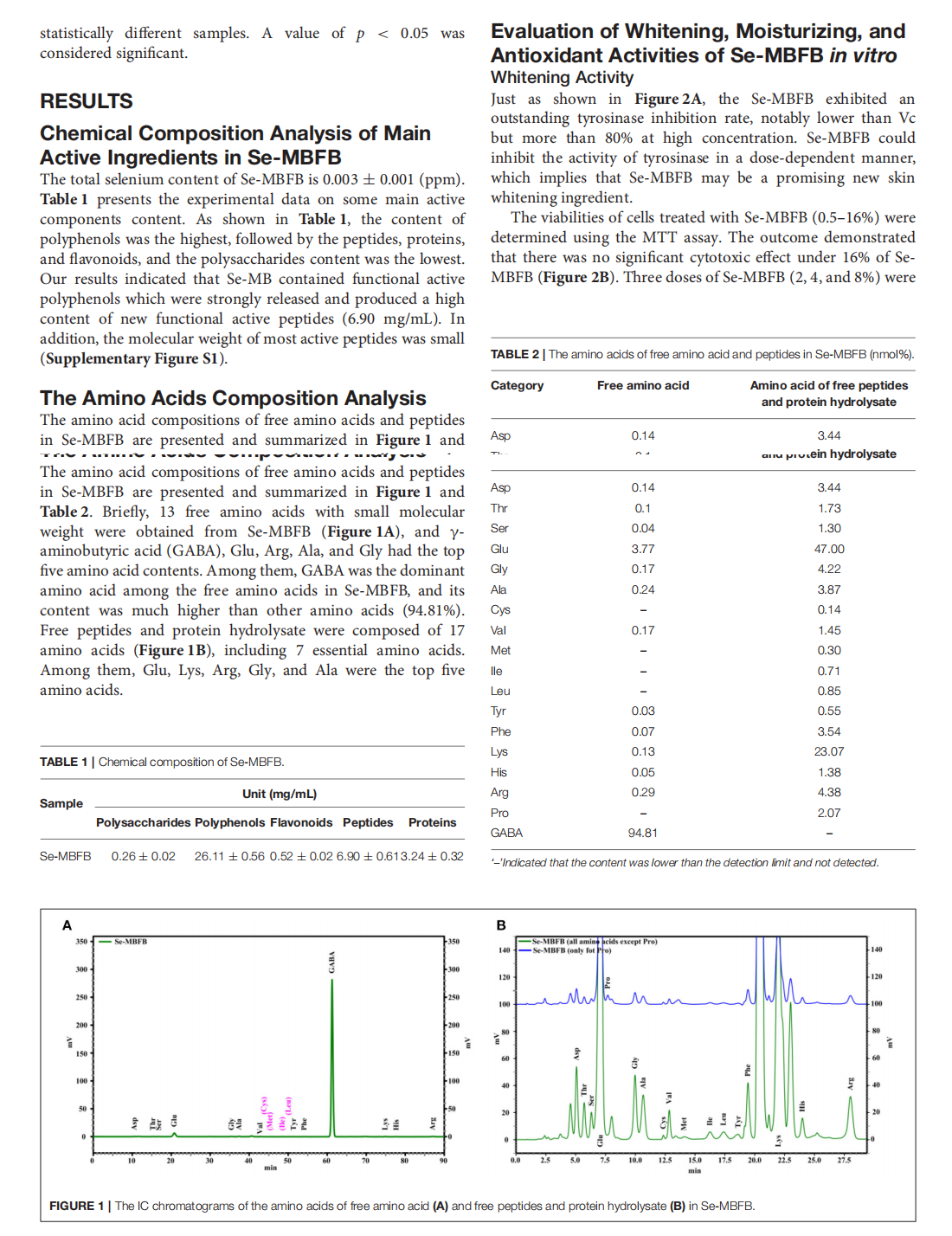
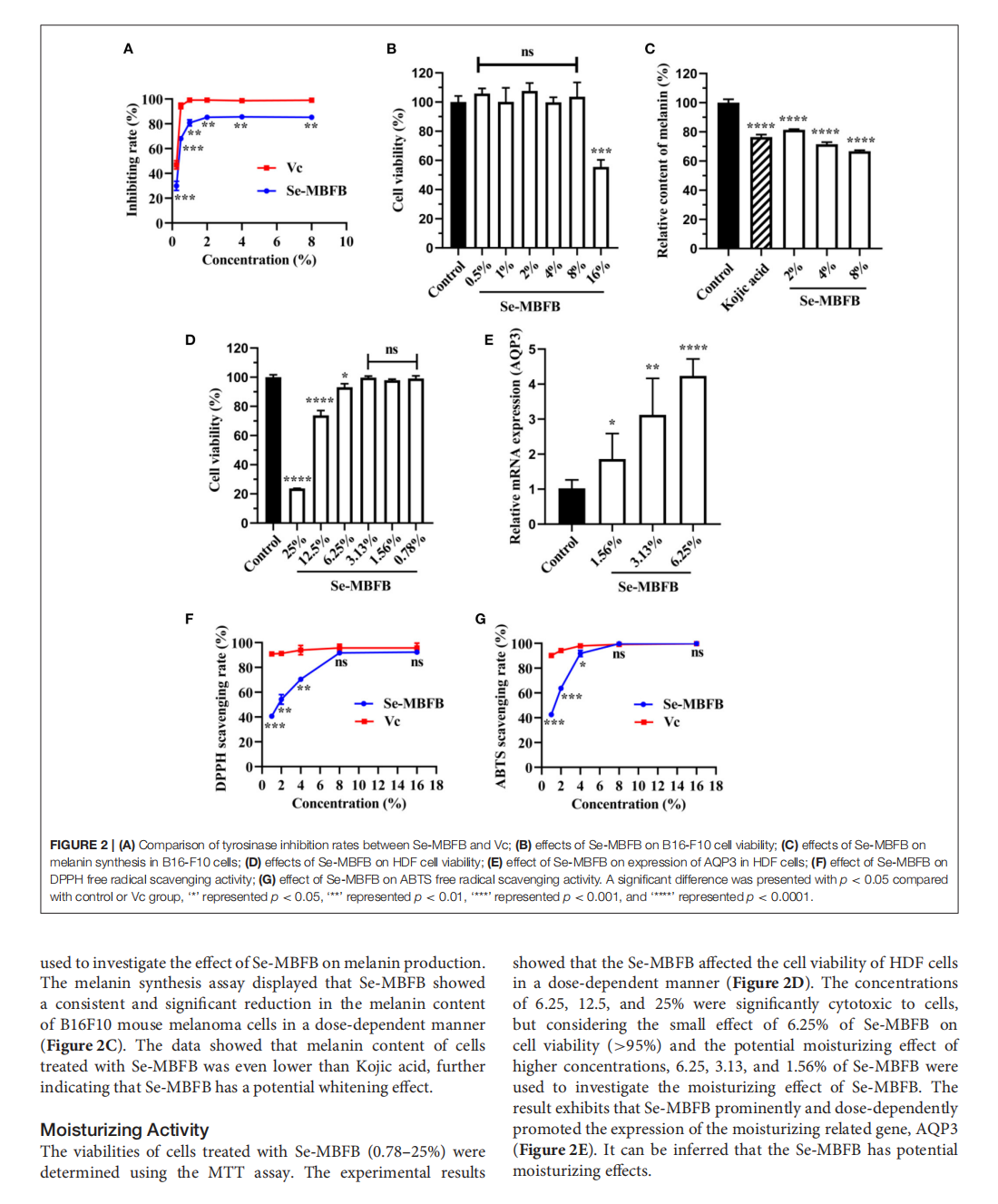

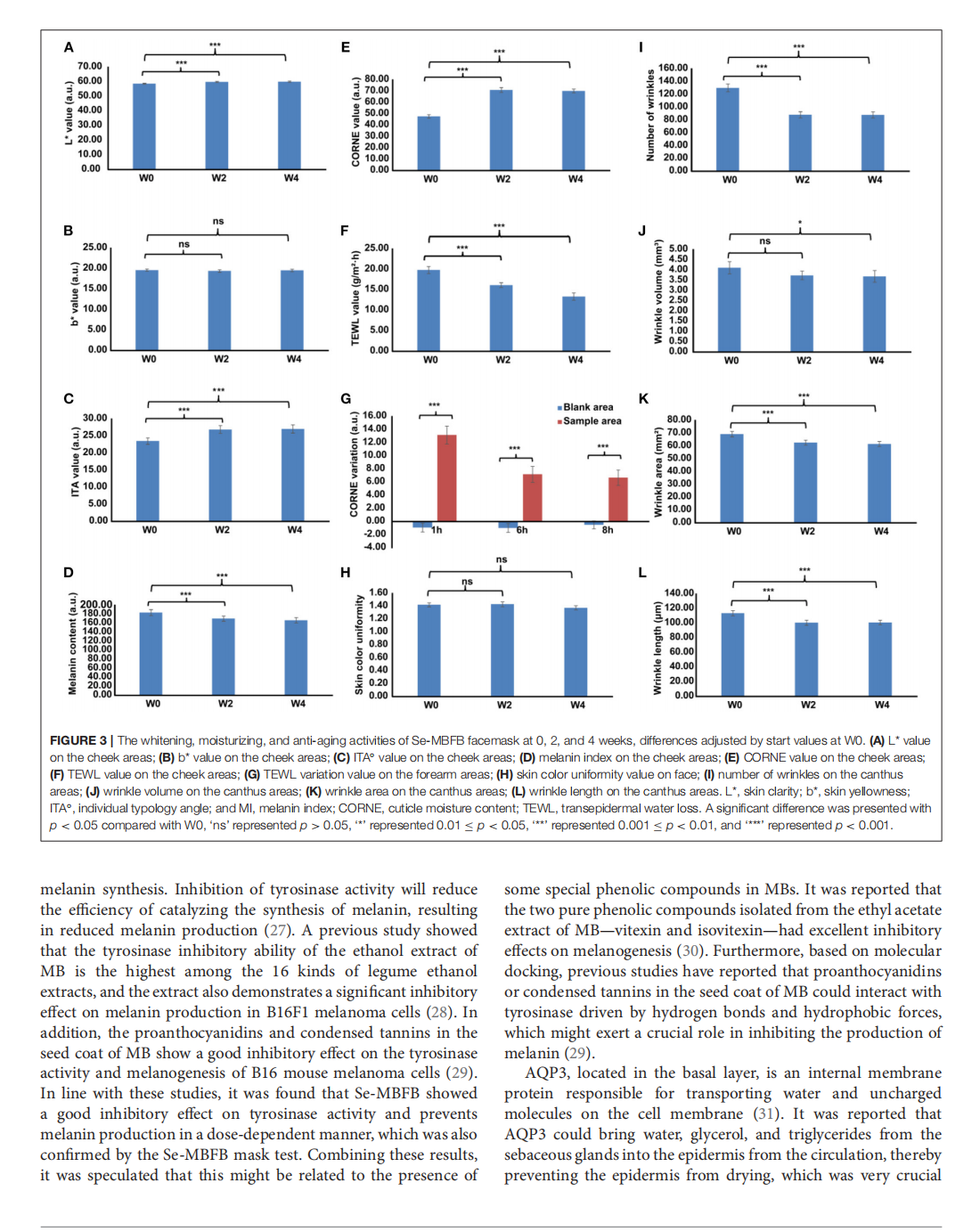
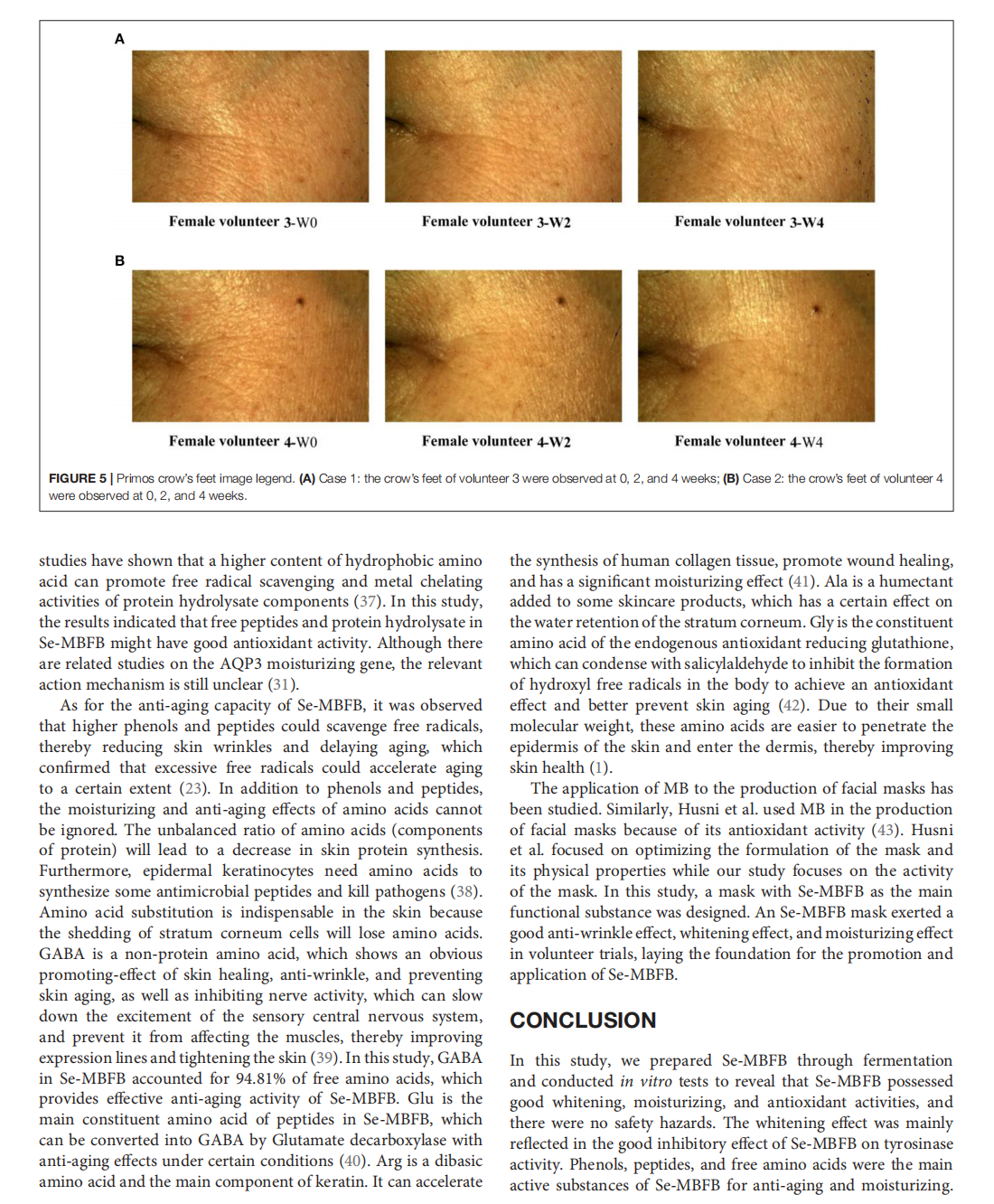


This article is excerpted from the《Frontiers in Nutrition》by Wound World

- 星期四, 15 1月 2026
Bridging clinic to home: domestic devices in dermatological diagnostics and treatments







This article is excerpted from the 《Frontiers in Digital Health》 by Wound World
