Issue: Volume 53 - Issue 10 - October, 2007
Index: Ostomy Wound Manage. 2007;53(10):50-58.
Login or Register to download PDF
Soft tissue breakdown disables an estimated 1.3 to 3 million patients per year in the US.1,2 The total cost of associated healthcare is $8 billion per year.3 Miller and Delozier4 estimated that in 1992, treating patients with pressure ulcers cost $1.335 billion (average charge was $21,675). It is universally accepted that a reduction of pressure between the body and the support surface interface will reduce or prevent the occurrence of pressure ulcers. Landis’5 observation (using a microinjection method) that 32 mm Hg capillary pressure is a threshold above which pressure ulcer occurs is an often-used industry guideline for testing the effectiveness of a support surface. The healthcare industry and healthcare providers have been active in developing new products and treatment guidelines to reduce the occurrence of pressure ulcers. Currently, more than 200 support surfaces6 are available that aim to either redistribute or reduce interface pressures to below the 32 mm Hg threshold. These pressure-relieving and pressure-reducing products have been widely applied with the hope of reducing pressure ulcer frequency. The effectiveness of support surfaces is usually tested by their ability to reduce interface pressures in healthy populations. Many studies7-17 have been published by researchers, clinicians, and the support surface industry to aid healthcare staff in choosing the right support surface for their patients. These studies mostly evaluate or compare the different support products using interface pressure measurements alone, mainly because this method is readily available, easy to use, non-invasive, and reasonably accurate.9,10,11,13
Despite such efforts, data from a 2003 nationwide inpatient sample18 indicate that the rate of hospital stays related to pressure ulcers has increased 63% from 280,000 cases in 1993 to 455,000 cases in 2003; of these patients, 72% with pressure ulcers were 65 years of age and older and 19% were between 45 and 64 years of age.18 The five most common conditions for pressure ulcer-related hospital stays were septicemia, pneumonia, urinary tract infection, aspiration pneumonitis, and congestive heart failure. Common concomitant conditions for patients admitted primarily for pressure ulcers were paralysis, spinal cord injury, substance abuse, malnutrition, multiple sclerosis, stroke, and senility.18 The average charge for treating pressure ulcers was nearly $37,800 per person for a total cost of approximately $17.2 billion in the year 2003. The prevalence (the number of patients with ulcers divided by the number of patients at risk for ulcers) and incidence (the number of patients who develop a pressure ulcer after admission to a hospital) of pressure ulcers in acute care settings has remained steady at approximately 15.3% and 7.6%, respectively, for the period 1999 to 2004.19 This has led many researchers to revisit the role played by pressure in the development of pressure ulcers and the effectiveness of pressure-relieving support surfaces.
Due to the paucity of other relevant data, healthcare providers continue to rely on research studies that primarily address interface pressure to select pressure-relief support devices. They fail to adequately consider all the other factors contributing to the formation of ischemic necrosis – ie, temperature, moisture, duration of the applied load, atrophy, and posture. As a result, support surfaces provide mixed, or in some cases, no benefits for patients. Correlating the interface pressure measured for various pressure-relieving support surfaces with relevant prevalence or incidence information will be useful in understanding the role of pressure in reducing pressure ulcers and the effectiveness of pressure-relieving systems.
The objective of this overview is to critically review the literature in order to analyze the relationship between interface pressure and pressure ulcer occurrence at various anatomic sites and to briefly review the role of other factors in the development of pressure ulcers.
Methods
A MEDLINE® search of English-language literature published from 1975 through December 2006 was conducted to identify publications that addressed either interface pressures on pressure-relieving support surfaces or pressure ulcer prevalence in the general population and spinal cord injured (SCI) patients. Criteria for article selection included: reports of interface pressure or prevalence values for at least three anatomic locations on the body, identification of support surfaces tested, and presentation of data in an easily analyzable format (tables or graphs). The search identified eleven publications7-17 (see Table 1) that reported interface pressures and six19-24 (see Table 2) that reported pressure ulcer prevalence information at various anatomic sites for either the general population or SCI patients.
 To gauge the relationship between reported interface pressure and ulcer prevalence, the pressure ulcer prevalence information and tissue interface pressure reported (summarized in Table 1 and Table 2 and shown in the Figures) was weighted based on the reported sample size and then averaged for each anatomic location. This weighted average was used to perform correlation analysis between interface pressure and prevalence of pressure ulcers at various anatomic locations. The sample sizes for studies reporting interface pressures were small compared to the prevalence studies.
To gauge the relationship between reported interface pressure and ulcer prevalence, the pressure ulcer prevalence information and tissue interface pressure reported (summarized in Table 1 and Table 2 and shown in the Figures) was weighted based on the reported sample size and then averaged for each anatomic location. This weighted average was used to perform correlation analysis between interface pressure and prevalence of pressure ulcers at various anatomic locations. The sample sizes for studies reporting interface pressures were small compared to the prevalence studies.
Many inconsistencies and a lack of standardization were noted among the studies analyzed. For studies reporting interface pressure, the interface pressures for each location over all surfaces tested were averaged because not enough information about the tested support surfaces or methodology was available to more reliably compare studies. Also, the intention was not to evaluate or compare support surfaces, but rather to assess the relationship between various pressure-relieving support devices and the prevalence of pressure ulcers in the acute care setting. To further investigate the role of pressure on pressure ulcer prevalence, anatomic locations were selected for which both interface pressure and prevalence information was available for the general as well as the SCI patient population.
Results
The occurrence of pressure ulcers and the corresponding measurements of body interface pressures at different anatomic regions are highly variable depending on tissue health, thickness, support characteristics, and method of measurement. Few papers reported interface pressure at more than three locations; the locations most reported are the sacrum, trochanter, and heels. Only one paper7 reported data on more than two locations in the SCI population. Figure 1 shows the distribution of pressure ulcers at various anatomic locations for the general population and SCI patients. Except for occiput, elbow, and heels, the distribution of pressure ulcers was similar between the two populations.

The weighted average interface pressures at various anatomic locations as reported in the publications listed in Table 1 are shown in Figure 2. As can be expected, the interface pressure for healthy volunteers and the general population is much lower than in the SCI population25 except for the occiput. A correlation analysis was performed between the interface pressures and the prevalence for four locations (occiput, sacrum, ischium, heel) where data were available for both the general population and SCI patients (see Table 3). A slightly negative correlation was found for both the general population and SCI patients. Both groups had 31% of occurrence of distribution for pressure ulcers at the sacrum but SCI patients had a 70% higher interface pressure compared to the general population. Regarding heel ulcers, the general population had a much higher occurrence (27% versus 16%) than SCI patients, while the reported interface pressure was actually lower (61 mm Hg versus 65 mm Hg).
Discussion
The data presented have to be interpreted with caution due to the limitations and shortcomings of using data from multiple sources, years, surfaces, patient groups, investigators, and measurement technologies. Missing data, small sample sizes, and lack of consistency in methods used further limit data interpretation. However, until data from a randomized controlled study with continuous or frequent measurements of interface pressure at various anatomic sites and collection of confounding factors and ulcer incidence data are available, the information presented here may help develop new research theories and increase understanding of the role of pressure redistribution in the development of these ulcers. Despite the limitations discussed, the analysis presented suggests that no direct or positive relationship exists between interface pressure and the distribution of pressure ulcers at various anatomic locations. This observation suggests that other factors, such as support surface microenvironment, influence ulcer formation at these anatomic sites.
More than 100 biomechanical and pathophysiologic risk factors for ischemic skin and soft tissue necrosis have been identified.1Of these, external pressure has been the most frequently discussed risk factor in the formation of ulcers. Other primary stress factors associated with ulcer formation are shear,26 friction, and the resulting deformation of soft tissues. Secondary or environmental factors important in bed immobility are temperature, moisture, duration of the applied load, atrophy, and posture. These factors influence tissue quality by reducing the strength and the rigidity of soft tissues and increase the friction coefficient of the skin.
Shear. Shear stress in soft tissues is generated by the tangential force component of body weight on the contact area externally and by the parallel and opposite tangential force on the bony prominence internally. Tangential forces acting on the skin develop shear stress in the tissues through friction and cause the tissue layers to slide against each other. The amount of sliding depends on the looseness of the connective fibers between tissue layers. If the fibers are tight, the skin and subcutaneous tissue will be subjected to higher shear stress; if the fibers are loose, more sliding than shear stress will occur. Shear stress is reduced by decreasing tangential force and increasing contact area. Loose covers and increased immersion in the support medium also increase contact area and further reduce shear stresses. When shear-induced tissue sliding occurs, blood vessels approaching the skin surface perpendicularly will bend and occlude at the connective layers between the tissue planes. Thus, shear will increase the effect of pressure in reducing flow through the blood vessels.27 Conversely, if shear stress is reduced, tissues can tolerate higher pressures without blood flow occlusion.
Friction. Friction describes the ability of a surface to prevent motion due to forces tangential to the contact area. The tangential or frictional force depends on the perpendicular force and the friction coefficient independent of the contact area. When the cover of the support surface is designed to allow movement over its foundation, slippage occurs between the cover and the bed and not within the tissue layers; thus, tension (stretching) in the skin and blood vessel occlusion are decreased due to a lower friction coefficient (see Figure 3). The tangential force is reduced most effectively by decreasing the friction coefficient on the support surface. The effect of a high friction coefficient is shown in Figure 4. The effectiveness of properly inflated air, water, and viscous fluid or gel supports is based on these principles. Combinations of these biomechanical principles are commonly used in modern support surfaces to create a better physical environment for tissue survival.
Temperature. Elevated body temperature raises the metabolic activity of tissues by 10% for every one degree Celsius of temperature increase, concurrently increasing the need for oxygen and an energy source at the cellular level.28 If the patient has impaired circulation from local pressure and shear, tissues will starve and release lysozymes, inducing autodigestion of cytoplasma and reducing skin integrity. Metabolic activity may cease from lack of energy and the accumulation of waste products, compromising skin integrity.29 It has been shown in animal studies30 that pressure-induced tissue injury accelerates with increasing body temperature (see Table 4). Increases in skin temperature also induce the sweat response, increasing the potential for moisture to accumulate at the skin-support interface.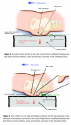
Moisture. Moisture from sweating or incontinence will hydrate the skin, dissolve the molecular collagen crosslinks of the dermis, and soften the stratum corneum (maceration). Skin maceration reduces stiffness, threatening the near complete loss of connective tissue strength and the erosion of the dermis under the action of shear forces. Another result of skin hydration is the rapid increase of the epidermal friction coefficient, which promotes adhesion of the skin to the support surface and increases shear, easy sloughing, and ulceration. Compounding the destructive effect of stress, excess moisture dilutes the skin’s natural acidity, reducing antibacterial properties of the epidermis and increasing the threat of sepsis.29,30
Two excellent technologies for controlling the microclimate at the skin-support surface interface are available. Low- and high-air-loss31 and air-fluidized support systems are designed to reduce stress and temperature, facilitate moisture evaporation, and prevent heat accumulation at the skin-support surface interface. The evaporation of 1 Kg of water from the skin at the support surface will remove 580 Kcal of heat from the body through the “latent heat of vaporization.”28 Thus, for an average person with 1.8 m2 skin surface and water loss of 26.7g/m2/hour, the cooling power is 27.9Kcal/hour. With proper design and nursing care aimed at maintaining a physiologic water balance, dynamic air loss support surfaces are able to control interface pressure, shear, and skin temperature and moisture.
Pressure. Pressure stress in soft tissues arises from the force component perpendicular to the external contact area and from body weight acting through the nearest bony prominence. In the design of support surfaces, the objective is to increase contact area by greater “immersion,” allowing the body to sink deeper into the surface, distributing the force and reducing the pressure. Cyclic transfer of weight from high-pressure areas is the main principle underlying alternating pressure support systems but resultant alternating pressure gradients, which are related to shear stress, may damage adipose tissues and capillaries that lack tensile strength.26,27
Pressure and shear stress have a similar effect on tissues; both reduce blood flow and, subsequently, tissue perfusion.31 A simple reduction of tissue stretching (shear) can nearly double the ability of tissues to withstand pressure without the development of ischemia.27 With this fact in mind, many support surfaces are designed to reduce shear, improving the weight-bearing tolerance of soft tissues.32
Patients most affected. Pressure ulcers are most prevalent in two groups of patients – those with neurological disorders (SCI, stroke, head trauma) and the elderly.2,21,23,24 The similarity is not surprising because the loss of muscle strength, skin and muscle protein, and muscle mass as a result of aging is similar to the losses observed in patients with neurological disorders. Skin changes in mechanical strength and susceptibility to external loads and alterations in subcutaneous tissues that accompany aging and neurological disorders indicate a significant reduction of tissue viability from normally innervated tissues.
Conclusion
The analysis methods employed in this assessment were limited because few studies33,34 have reported both pressure and corresponding pressure ulcer incidence or prevalence at various anatomic sites. Available control data lack standardization (no standard “standard” mattress exists) and studies tend to compare mattresses with different features from different manufacturers. As a result, a direct comparison between most studies is not possible or accurate. Unfortunately, healthcare providers rarely have data other than interface pressure to evaluate and select support surfaces for their patients. However, the National Pressure Ulcer Advisory Panel (NPUAP) is currently developing standardization methodology for support surfaces through its Support Surface Standardization Initiative (S3I).35 This analysis of published data, despite its limitations, failed to demonstrate a direct or positive relationship between measured interface pressures and the occurrence of pressure ulcers at various anatomic locations. In the absence of more definitive data, these observations suggest that clinicians need to consider the ability of a pressure-relieving support surface to not only redistribute pressure but also to control microenvironment.


