Keywords
negative pressure wound therapy
Issue: Volume 57 - Issue 4 - April 2011
Index: Ostomy Wound Manage. 2011;57(4):44‚54.
Login or Register to download PDF
Abstract
Currently available research suggests that negative pressure wound therapy (NPWT) creates a moist wound healing environment, drains exudate, reduces tissue edema, contracts the wound edges, mechanically stimulates the wound bed, and influences blood perfusion at the wound edge, which may lead to angiogenesis and the formation of granulation tissue.
Although no clear evidence is available that NPWT accelerates wound healing compared to other interventions or that one form of NPWT is better than another, preclinical research suggests that the most commonly used dressings, level of negative pressure, and application mode (continuous, intermittent, or variable) may not be optimal for all patients. To summarize available literature related to these NPWT choices, pertinent literature published between 2005 and 2010 was reviewed. Preclinical study results suggest that the maximal biological effect of NPWT at the wound edge often can be achieved at -80 mm Hg and that foam dressings may be advantageous for large defect wounds, whereas gauze dressings may be more suitable for smaller wounds or when scar formation or pain is a concern.
Preclinical research results also suggest that intermittent or variable pressure application has a better effect on granulation tissue formation than continuous application. The variable pressure mode maintains a negative pressure environment at lower pressure settings without dramatic fluctuations inherent to intermittent (on-and-off) pressure. Prospective, controlled clinical studies are needed to compare NPWT to other advanced wound care protocols of care and to ascertain the effect of various NPWT methods and regimens on outcomes of care.
Potential Conflicts of Interest: The work was supported by the Swedish Medical Research Council, Lund University Faculty of Medicine, the Swedish Government Grant for Clinical Research, Lund University Hospital Research Grants, the Swedish Medical Association, the Royal Physiographic Society in Lund, the Åke Wiberg Foundation, the Anders Otto Swärd Foundation/Ulrika Eklund Foundation, the Magn Bergvall Foundation, the Crafoord Foundation, the Anna-Lisa and Sven-Erik Nilsson Foundation, the Jeansson Foundation, the Swedish Heart-Lung Foundation, Anna and Edvin Berger’s Foundation, the Märta Lundqvist Foundation, Lars Hierta’s Memorial Foundation, and Prospera (Fort Worth, TX).
Negative pressure wound therapy (NPWT) is increasingly used to treat hard-to-heal wounds and has been shown to have the potential to improve healing outcomes in many wound types, including orthopedic trauma (case series),1 soft tissue trauma (prospective, randomized study),2 skin grafts (case series),3 flaps, pressure ulcers (prospective, randomized study),4 venous leg ulcers (randomized, controlled trial [RCT]),5 vascular surgery wounds, diabetic foot ulcers (RCT),6 burns (case series),7 wound dehiscence following abdominal (retrospective review)8 and thoracic (retrospective review)9 surgery, and surgical infections (case series).10

Before negative pressure is applied, the wound is filled with a porous material — currently, gauze11 or polyurethane foam with a porous structure is used as wound filler. The pressure applied by the vacuum pump is propagated through this filler to the wound bed, leading to the removal of exudate. The wound is sealed with an adhesive plastic drape. A drain is used to connect the wound filler to the vacuum source (see Figure 1). NPWT dressings normally are changed every 2 or 3 days.12
Vacuum pumps have been designed to specifically serve NPWT needs, with special attention to safety issues. NPWT device features include alarms for leakage (to ensure the wound does not dry out if it is not sealed correctly) and occlusion (to ensure the negative pressure environment is not compromised). The fluid-collection canister can easily be inspected with regard to drainage and bleeding
In recent years, intensive research has been conducted to investigate the biological effects of NPWT on the wound bed and to find ways to optimize use of this technology. The purpose of this overview is to summarize: 1) recent publications regarding the choice of negative pressure level, the type of wound filler to be used, and the mode of NPWT delivery (continuous, intermittent or variable); and 2) methods of varying these individual parameters to optimize care.
Literature Search
A literature search was performed using the PubMed database. Search terms included negative pressure wound therapy, vacuum-assisted closure, wound filler, gauze, foam, variable, intermittent, continuous, and pressure level. English-language articles with publication dates between 2005 and 2010 were reviewed. The authors also searched their personal journal article collections.
Physiological Mechanisms
Overview. After more than 10 years of intensive research and clinical application, NPWT has been found to create a moist wound healing environment (review article),13 drain exudate (clinical case series and controlled studies in experimental wound models),14-16 reduce tissue edema (controlled studies in experimental wound models),17 contract wound edges (clinical case series and controlled studies in experimental wound models),14-16 mechanically stimulate the wound bed (experimental wound models and computer simulation),18-20 alter blood flow in the wound edges (healthy human and experimental wound models),15,21-23 and stimulate angiogenesis (clinical case series and controlled studies in experimental wound models)24,25 and the formation of granulation tissue (experimental wound models).15 The biological effects of NPWT are represented in Figure 1. In clinical studies on patients, NPWT did not reduce the bacterial burden in the wound,26,27 but it is speculated that the treatment may offer protection against infection because the wound is sealed. The type of cotton gauze used as a NPWT dressing is impregnated with polyhexamethylene biguanide (PHMB) (Kerlix AMD, Covidien, Mansfield, MA) that may provide antimicrobial control.28
Summarizing NPWT mode of action, the Agency for Healthcare Research and Quality (AHRQ) report on NPWT29 states that, “Negative pressure wound therapy (NPWT) applies a localized vacuum to draw the edges of the wound together while providing a moist environment conducive to rapid wound healing.” This is also the empirical clinical experience when using NPWT — ie, the wound remains moist while excessive fluid is removed. However, a RCT comparing traditional moist wound therapy and NPWT has not yet been conducted.
Pressure transduction and wound drainage. The negative pressure is created by a vacuum pump and is delivered to the wound by means of drainage tubing. Ultimately, the negative pressure is propagated through the wound filler to the wound bed. As shown in a porcine study,19 the pressure distribution is similar for gauze and foam. It should be noted that the pressure affects only the tissue in direct contact with the wound filler; as shown in an experimental wound model,30 it does not extend to deeper structures. Therefore, it is important to place the wound filler in direct contact with all areas of tissue in which an effect is desired. As discovered empirically in the clinical setting, if fluid is to be drained from a deep wound pocket, it may be concluded that the entire pocket needs to be in contact with the filler. When treating complicated wounds with deep pockets, the wound filler must be carefully positioned — eg, by using a cotton swab (see Figure 2). Under such conditions, it may be easier to use gauze because it can be adapted to the shape of the wound.31 Results of a preclinical study (porcine wound model)32 have shown that, compared to foam, gauze facilitates dressing changes and reduces the risk of the wound filler becoming attached to the tissue and remaining in the wound, which is of special importance in wounds with deep pockets that are difficult to inspect.
The suction pressure leads to exudate removal from the wound and prevents fluid retention in deeper parts of the wound. In chronic wounds, necrotic tissue and slough tend to continually accumulate due to underlying pathogenic abnormalities that alter the biochemical and cellular environment.33 The accumulation of necrotic tissue or slough in a wound is known to promote bacterial colonization and may prevent complete repair of the wound.34 However, it is not clear whether elevation of, for example, matrix metalloproteinases, is responsible for delayed wound healing or merely the result of other changes occurring within the chronic wound — ie, there is an association but not necessarily a causal relationship. Similarly, it has been shown in clinical case series25,35 that decreases in some metalloproteinases are associated with wound healing and these decreases are known to occur during NPWT.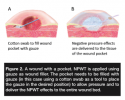
Edema. The inflammatory reaction to a wound causes tissue edema, which in turn increases interstitial pressure and reduces blood flow. As a result, the supply of oxygen and nutrients to the wound edge is reduced, weakening resistance to secondary infection and delaying healing. Thus, in order to facilitate wound healing, it is important to reduce tissue edema. NPWT causes compression of the tissue closest to the surface of the wound, which is believed to reduce interstitial edema.36 Wound edge tissue compression also facilitates the opportunity to tamponade bleeding during surgery, as described in a case report37 of a patient with mediastinal bleeding after cardiac surgery. Surgical sponges were placed around the heart and covered with a transparent drape and suction (-15 mm Hg) was applied to the pleural cavities. In response, the drape was sucked inward over the surgical sponges and into the mediastinum. Bleeding promptly decreased due to the tamponade effect between the mediastinal tissue and the surgical sponges.
Mechanical effects on the wound edges.One of the effects of NPWT is the contraction of the wound edges, which leads to tissue remodeling that facilitates wound closure. It also has been found in experimental wound models20 that the wound tissue and the wound filler material interact on a microscopic level (microdeformation). The wound bed is drawn into the pores of the foam or in-between the threads of the gauze. These mechanical effects affect the cytoskeleton of the cells1 and initiate a cascade of biological reactions that result in granulation tissue formation and subsequent wound healing.38
Several in vitro studies39-42 have been conducted that demonstrate the role of mechanical tension and shear on the ability of cells to develop and maintain a synthetic phenotype that enables formation of extracellular matrix and fibrous structural constituents. Mechanical deformations are known to promote angiogenesis by inducing formation of capillary sprouts (mechanical tension) or endothelial cell signaling via mechanical shear stress mechanisms.43-45 Given the in vitro and in vivocorrelations between mechanical tension, compression, and formation of granulation tissue, the ability to reliably control the state of stress and strain in the wound bed becomes exceedingly important.39-42
Effects on blood flow in the edges of the wound. Most studies on periwound blood flow changes in response to NPWT have been conducted in porcine wound models. The effects on blood flow resulting from NPWT are local and vary depending on the distance from the wound edge (see Figure 3).22 The superficial blood flow close to the edge of the wound (within about 5 mm) decreases, whereas it increases farther away from the wound edge (about 25 mm).22,23,46 This combination of increased and decreased blood flow is theorized to be advantageous in the wound healing process because increased blood flow may lead to improved oxygen and nutrient supply to the tissue, as well as improved penetration of antibiotics and the removal of waste products. The mechanism behind the increase in blood flow has not yet been identified, but it has been speculated that the negative pressure causes a force in the tissue that opens up the capillaries, increasing flow. As has been shown both in vitro36(in soft plasticene and processed meat) and in vivo (in human wounds),47 blood flow reduction occurs in response to the negative pressure compressing the tissue surface. When tissue perfusion is reduced, angiogenic factors are released to stimulate the formation of new blood vessels.48 This promotes granulation tissue formation and, ultimately, wound healing.
Formation of granulation tissue. Granulation tissue is the combination of small vessels and connective tissue that forms in the wound bed. It provides a nutrient-rich matrix that allows epidermal cells to migrate over the bed of the wound. In a porcine wound model,15 NPWT using foam accelerated the formation of granulation tissue compared to standard saline-moistened gauze (without NPWT). It is believed that the introduction of the wound filler may lead to the release of various chemotactic factors. In response, neutrophils and macrophages migrate to the wound area.49The cells in the wound bed are transformed into fibroblasts, and a surrounding collagen matrix is formed.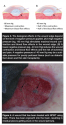
Individual Optimization by Pressure Settings, Dressings, and Mode
Until recently, the landmark porcine study by Morykwas et al15 published in 1997 has provided the basis for the way many clinicians use NPWT on patients. The study showed that -125 mm Hg is the optimal pressure with regard to local blood flow effects; however, details such as the optimal pressure setting for granulation tissue formation were missing and recent research has found that the settings for NPWT can be refined to optimize treatment. Today, negative pressure level, wound filler material (foam or gauze), and the mode (continuous, intermittent, or variable) by which the pressure is applied can be tailored to the individual. Results of in vivo research carried out during the past few years on the mechanisms involved have shown how the healing process can be influenced by varying these parameters. Much of this research has been carried out in pigs, but interestingly, experienced clinicians have come to the same conclusions when it comes to treating patients. This knowledge is now beginning to be employed in patient care to minimize complications such as ischemia and pain and to optimize outcome.
Negative pressure level. The most commonly used pressure level, -125 mm Hg, is based on 1997 research.15 However, more recent animal studies have shown that the maximal biological effects on the wound edges in terms of wound contraction,50regional blood flow,46 and the formation of granulation tissue32 are obtained at -80 mm Hg. A recent case report51 concurs that negative pressure levels <-125 mm Hg result in excellent wound healing. When using NPWT to treat poorly perfused tissue (eg, diabetic foot ulcers and thin skin transplants), ischemia may develop in the wound edges and the patient can experience pain during treatment.52,53 Thus, it may be advantageous to use negative pressure of -40 mm Hg in the treatment of sensitive, poorly perfused tissue, which is the level at which a porcine peripheral wound study46 showed that approximately half the maximal blood flow effect is achieved (see Figure 3). According to the same study,46 the use of negative pressure >-80 mm Hg is seldom necessary. However, another study on porcine peripheral wounds50 suggests that because exudate drainage may be improved, -125 mm Hg could be used for the first few days to treat high-output wounds, after which the negative pressure may be lowered once the amount of exudate lessens.
Wound filler. Polyurethane foam or gauze currently is used as the wound filler material in NPWT. Gauze-based NPWT differs from the wound therapy called “wet-to-dry gauze,” where moistened gauze is applied to the wound (without negative pressure) and allowed to dry out between dressing changes. Per nonpublished observations, both gauze and foam NPWT dressings provide a moist wound healing environment. Two animal studies and a case series32,53,54 have shown that the choice of wound filler material has considerable influence on the wound healing process as seen by the amount and characteristics of granulation tissue. The granulation tissue formed under foam is thick but fragile. When gauze is used, the newly formed tissue is thinner and more stable. In animal studies and clinicians’ empirical findings, hypertrophic granulation tissue eventually led to fibrosis and scar tissue, as well as to contractures (as seen in a case series on humans54); therefore, thick granulation tissue is undesirable when the cosmetic result is important or in cases where scar tissue may restrict movement — eg, over joints.31 In such cases, gauze is preferred. Empirical evidence indicates that foam may be a better choice in wounds where large amounts of tissue are desirable (eg, sternotomy wounds).
A porcine study55 where continuous NPWT at -125 mm Hg was used for 4 days showed that the contact between foam and the wound bed leads to ingrowth of tissue in the foam (no ingrowth is seen when gauze is used32). Thus, more force is required (and subsequently, more pain results56) when removing foam as opposed to gauze when changing dressings.32 Also, some of the foam material may be left in the wound and can later act as a foreign body and newly formed tissue may be torn when the foam is removed, resulting in nonselective mechanical debridement (see Figure 4). Attempts have been made to reduce pain by applying local anesthetics to the wound surface,57 and analgesics may be administered systemically before dressing changes to prophylactically address pain.58 Obvious advantages with impregnated gauze are the lack of tissue ingrowth and minimal pain during dressing change, as shown in a porcine experimental wound model.56
Ancillary dressings. When the clinician anticipates complications, a nonadherent wound contact layer dressing such as paraffin, mineral oil, or silicon may be placed over the wound bed beneath the wound filler.59,60 A wound contact layer also may be placed over vulnerable structures such as blood vessels or nerves60 as well as over the wound bed itself because it is believed to protect from ingrowth of granulation tissue into foam.32 In the clinical setting, the presence of a wound contact layer may reduce pain during dressing changes as has been reported in several case studies.58-61 However, studies in an experimental porcine wound model62 have shown that a wound bed under a nonadherent wound contact layer may form a somewhat thinner granulation tissue than a wound bed in direct contact with the wound filler. The reason for the difference in effect between a wound filler and a wound contact layer is that the structure of the material in the dressing in direct contact with the wound bed determines the effects of NPWT on the wound bed.32,62
NPWT mode: continuous, intermittent, and variable. Negative pressure is most commonly applied in the continuous mode. The pressure level is kept at a constant setting — eg, -80 mm Hg. Intermittent pressure therapy involves repeatedly switching on and off (for example, alternating between 0 mm Hg and -80 mm Hg), alternating between an atmospheric and subatmospheric pressure.15 Intermittent pressure therapy is not often used clinically because the sudden spiking of negative pressure changes causes the foam to expand and contract repeatedly over the granulation tissue, which can be painful for the patient. Variable pressure therapy was introduced to provide smooth cycling between two different levels of negative pressure (for example -10 mm Hg and -80 mm Hg), thereby maintaining the negative pressure environment throughout the therapy.63 Based on a study by Morykwas et al,15 treatment usually is applied in 5-minute cycles, although no study has yet been performed to determine the optimal timing. Figure 5 provides a schematic illustration of the different pressure modes. Porcine wound studies15,64 have shown that the amount of granulation tissue in the wound bed increases dramatically during both intermittent and variable NPWT compared to continuous therapy. This may be a result of mechanical stimulation of the wound bed (a massaging effect),64 as well as enhanced tissue oxygenation and angiogenesis because of enhanced blood flow.65 
Conclusion
The mechanisms by which NPWT may facilitate wound healing include the creation of a moist environment, drainage of exudate, reduction of tissue edema, contraction of the wound edges, mechanical stimulation of the wound bed, blood flow changes in the wound edges, and stimulation of angiogenesis and formation of granulation tissue. According to the Agency for Healthcare Research and Quality Technology Assessment report from 2009,29 no clear evidence is available that NPWT accelerates wound healing compared to other interventions or that one form of NPWT is better than another. In light of the lack of clinical comparisons, the latest preclinical study findings suggest that the choice of negative pressure level, the type of wound filler to be used, and the mode of NPWT delivery (continuous, intermittent, or variable) may affect the use of NPWT in clinical practice. These preclinical study findings can be summarized as follows:
• The maximal biological effect at the wound edge often can be achieved at -80 mm Hg. When treating wounds at risk of ischemia, it can be advantageous to limit the pressure to approximately -40 mm Hg.
• The characteristics of foam versus gauze as wound filler need consideration. Foam dressings create large amounts of granulation tissue, an advantage when rapid healing is desired. On the other hand, gauze induces the formation of a thinner but more stable layer of granulation tissue, which may lead to less scar formation. Gauze also has the potential advantage of no tissue ingrowth and less patient pain on dressing removal.
• The mode of pressure application can affect outcomes. Intermittent pressure application has a better effect on granulation tissue formation than continuous application but may cause pain. Variable pressure application has similar advantages but with reduced levels of pain.
While awaiting the results of controlled clinical studies, clinicians can use this information from preclinical research to individualize care by adjusting the amount and mode of pressure and selecting the most appropriate wound filler for the patient.
Dr. Borgquist is an anesthesiologist, Departments of Ophthalmology and Anesthesiology and Intensive Care; Dr. Ingemansson is an associate professor and thoracic surgeon, Department of Cardiothoracic Surgery; and Dr. Malmsjö is an associate professor and ophthalmologist, Department of Ophthalmology, Lund University and Skåne University Hospital, Lund, Sweden. Please address correspondence to: Associate Professor Malin Malmsjö, MD, PhD, BMC A13, SE-221 84 Lund, Sweden; email: 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。.


