

















This article is excerpted from the July 2022 | Volume 3 | Article 931331 Pathobiology of Antiaging Klotho by Wound World.
Gérald J. Prud’homme1,2 *, Mervé Kurt 2 and Qinghua Wang3,4
1 Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada,
2 Department of Laboratory Medicine, Keenan Research Centre for Biomedical Science, Unity Health Toronto, Toronto, ON, Canada,
3 Department of Endocrinology and Metabolism, Huashan Hospital, Shanghai Medical School, Fudan University, Shanghai, China,
4 Shanghai Yinuo Pharmaceutical Co., Ltd., Shanghai, China
Edited by:
Cátia F. Lourenço, University of Coimbra, Portugal
Reviewed by:
Mujib Ullah, Stanford University, United States Taylor Landry, East Carolina University, United States
*Correspondence:
Gérald J. Prud’homme 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。
Specialty section:
This article was submitted to Molecular Mechanisms of Aging, a section of the journal Frontiers in Aging
Received: 28 April 2022
Accepted: 06 June 2022
Published: 12 July 2022
Citation:
Prud’homme GJ, Kurt M and Wang Q (2022) Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations. Front. Aging 3:931331.
doi: 10.3389/fragi.2022.931331
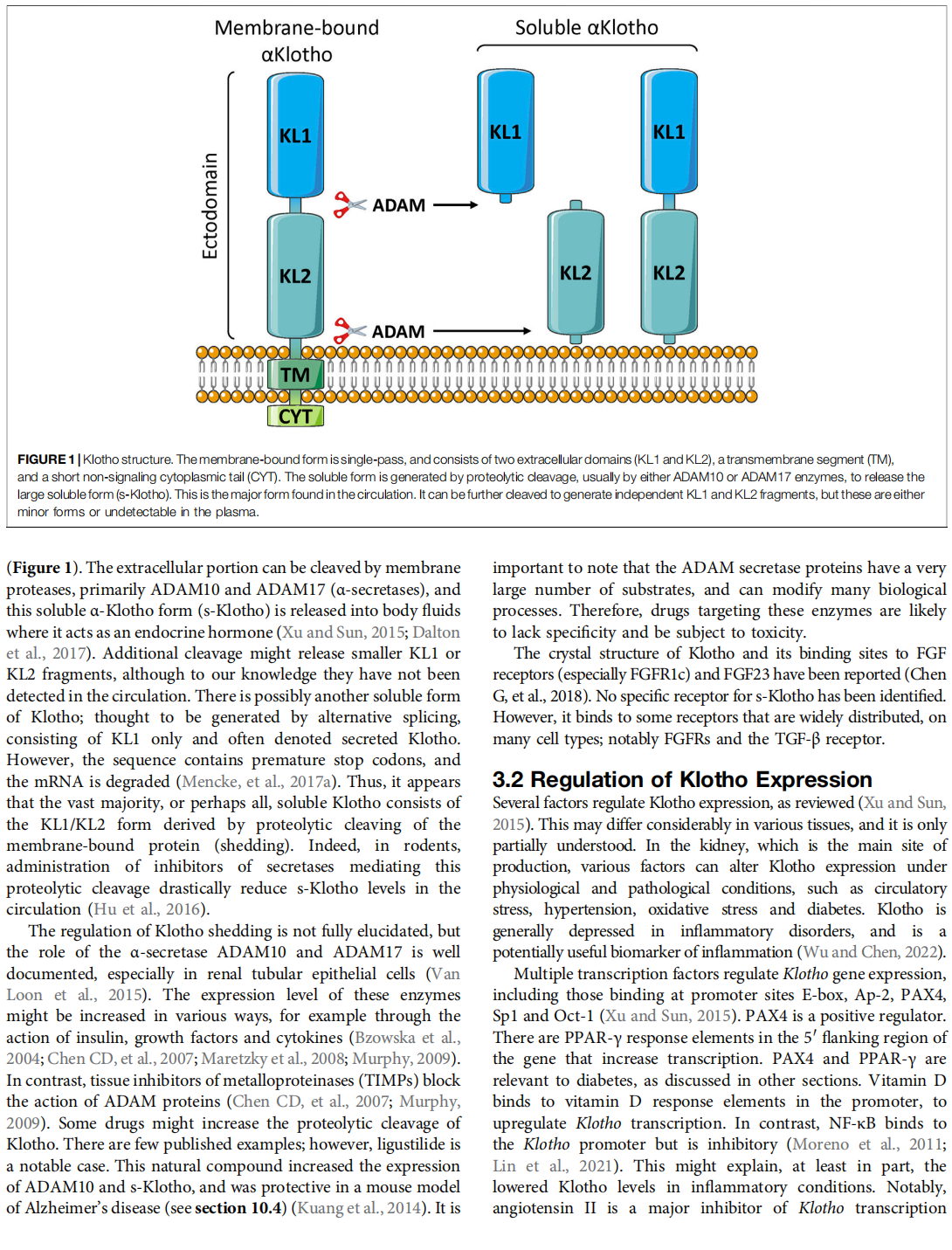
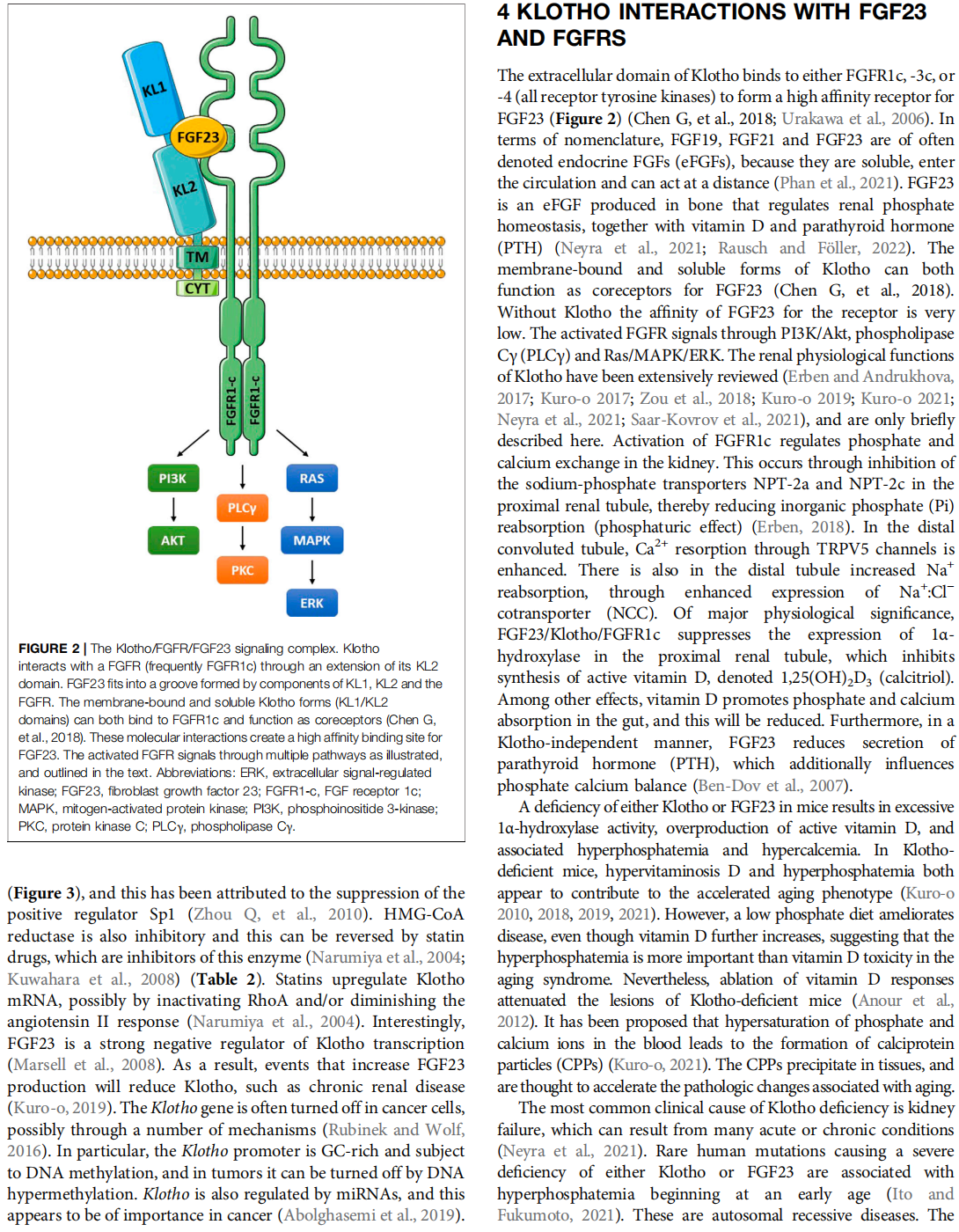
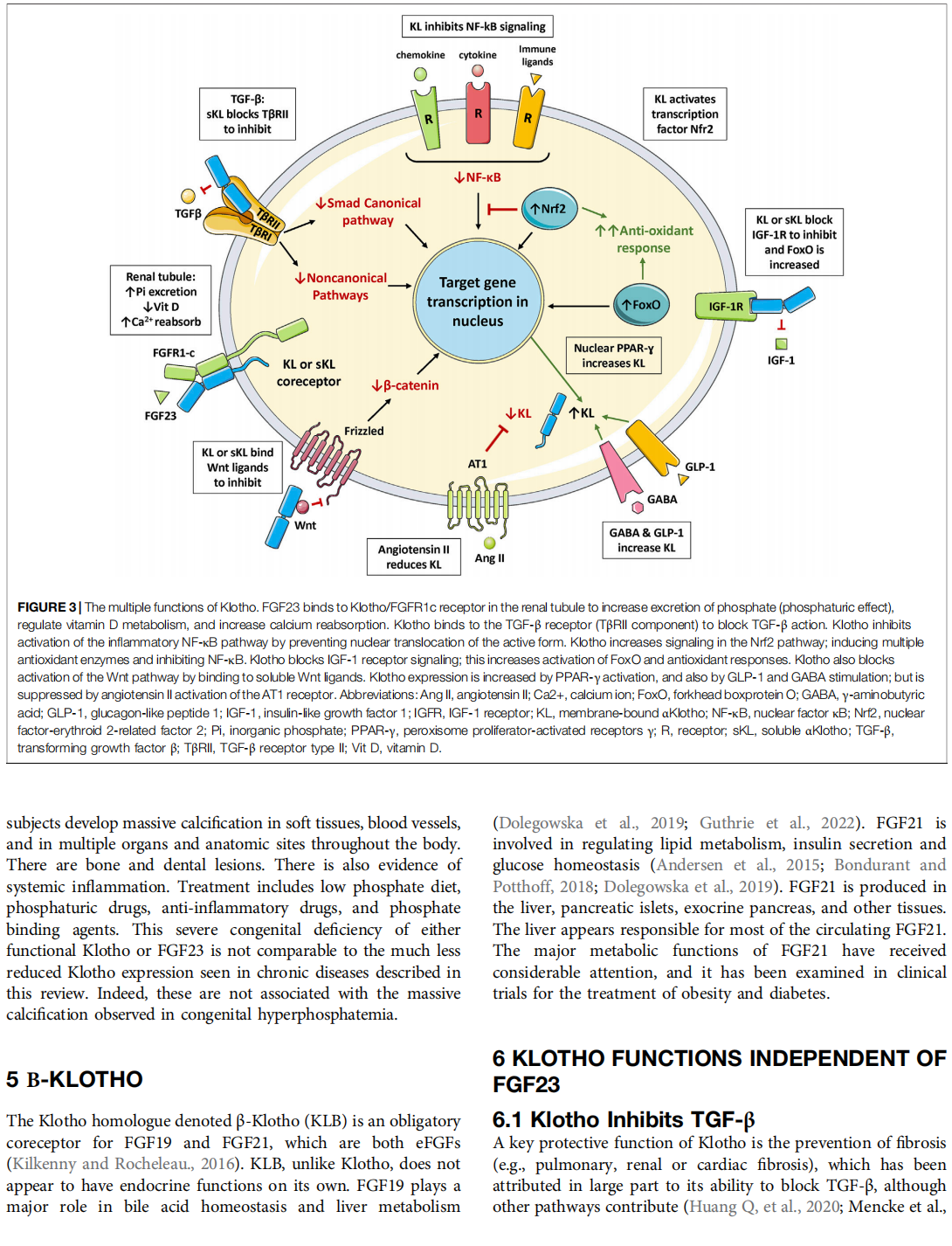
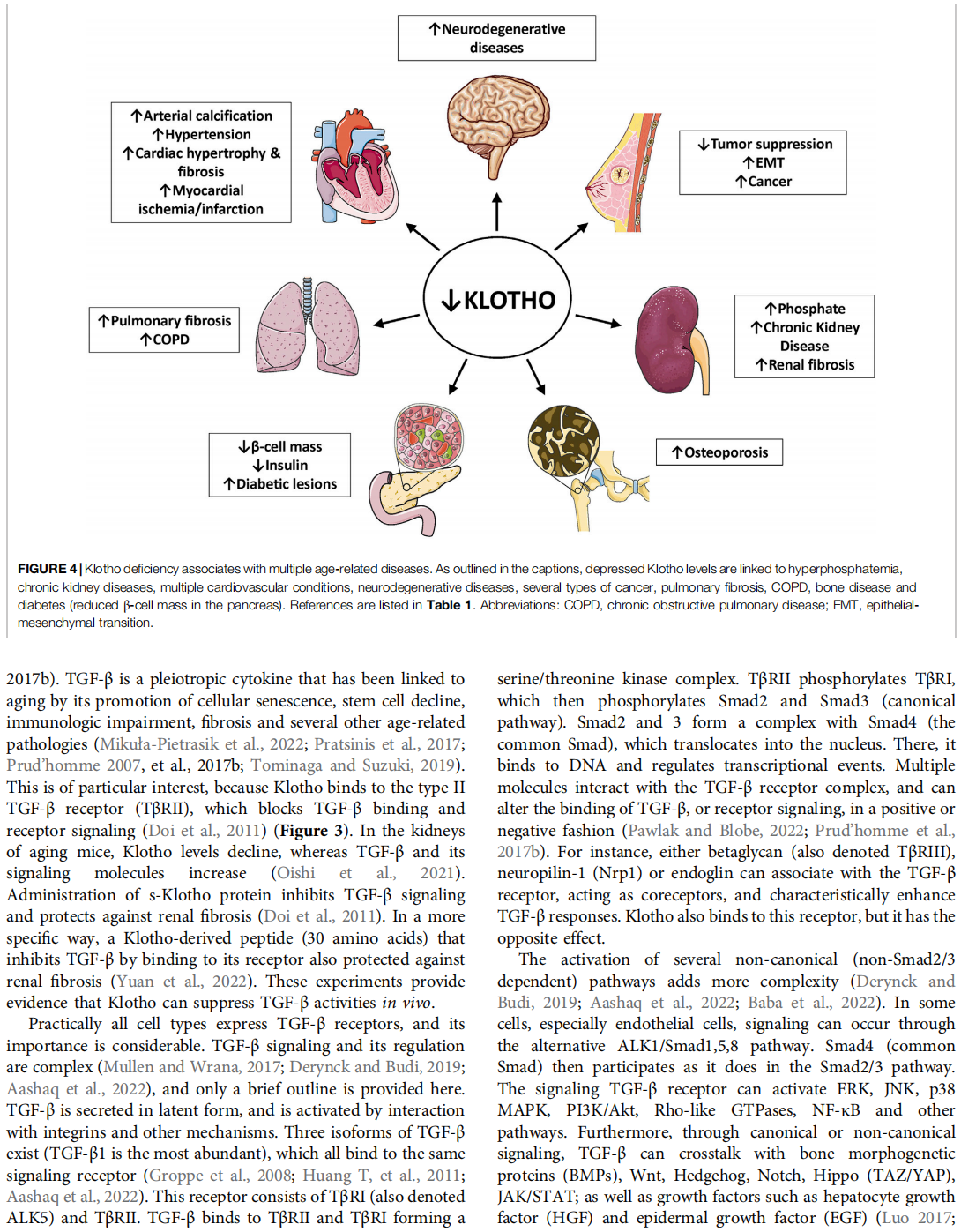
The α-Klotho protein (henceforth denoted Klotho) has antiaging properties, as first observed in mice homozygous for a hypomorphic Klotho gene (kl/kl). These mice have a shortened lifespan, stunted growth, renal disease, hyperphosphatemia, hypercalcemia, vascular calcification, cardiac hypertrophy, hypertension, pulmonary disease, cognitive impairment, multi-organ atrophy and fibrosis. Overexpression of Klotho has opposite effects, extending lifespan. In humans, Klotho levels decline with age, chronic kidney disease, diabetes, Alzheimer’s disease and other conditions. Low Klotho levels correlate with an increase in the death rate from all causes. Klotho acts either as an obligate coreceptor for fibroblast growth factor 23 (FGF23), or as a soluble pleiotropic endocrine hormone (s-Klotho). It is mainly produced in the kidneys, but also in the brain, pancreas and other tissues. On renal tubular-cell membranes, it associates with FGF receptors to bind FGF23. Produced in bones, FGF23 regulates renal excretion of phosphate (phosphaturic effect) and vitamin D metabolism. Lack of Klotho or FGF23 results in hyperphosphatemia and hypervitaminosis D. With age, human renal function often deteriorates, lowering Klotho levels. This appears to promote age-related pathology. Remarkably, Klotho inhibits four pathways that have been linked to aging in various ways: Transforming growth factor β (TGF-β), insulin-like growth factor 1 (IGF-1), Wnt and NF-κB. These can induce cellular senescence, apoptosis, inflammation, immune dysfunction, fibrosis and neoplasia. Furthermore, Klotho increases cell-protective antioxidant enzymes through Nrf2 and FoxO. In accord, preclinical Klotho therapy ameliorated renal, cardiovascular, diabetes-related and neurodegenerative diseases, as well as cancer. s-Klotho protein injection was effective, but requires further investigation. Several drugs enhance circulating Klotho levels, and some cross the blood-brain barrier to potentially act in the brain. In clinical trials, increased Klotho was noted with renin-angiotensin system inhibitors (losartan, valsartan), a statin (fluvastatin), mTOR inhibitors (rapamycin, everolimus), vitamin D and pentoxifylline. In preclinical work, antidiabetic drugs (metformin, GLP-1-based, GABA, PPAR-γ agonists) also enhanced Klotho. Several traditional medicines and/or nutraceuticals increased Klotho in rodents, including astaxanthin, curcumin, ginseng, ligustilide and resveratrol. Notably, exercise and sport activity increased Klotho. This review addresses molecular, physiological and therapeutic aspects of Klotho.
Keywords: aging, FGF23, hyperphosphatemia, klotho, IGF-1, NF-KappaB, TGF-beta, Wnt























This article is excerpted from the July 2022 | Volume 3 | Article 931331 Pathobiology of Antiaging Klotho by Wound World.
