Although the existence of sensitive skin has been challenged in the past, there is now a near-consensus that sensitive skin can be defined clinically (1) as the occurrence of abnormal stinging, burning, and tingling sensations (and sometimes as pain or pruritus) in response to multiple factors that may be physical (UV, heat, cold, wind), chemical (cosmetics, soaps, water, pollutants) and sometimes psychological (stress) or hormonal (menstrual cycle). Erythema is often but not always involved. Tests that may help to detect this skin problem include the stinging test, heat sensitivity test and capsaicin test. However, history-taking is obviously the most reliable method given that, by definition, skin sensitivity occurs in response to a variety of factors.
Sensitive skin is also known as reactive or overreactive skin, intolerant skin, and irritable skin. The term “reactive skin” seems preferable to “sensitive skin”, which may lead to confusion with skin sensitised due to an allergic disorder. Although the pathophysiology of sensitive skin remains unclear (2), the underlying mechanism is not immunological or allergic. Histological exams only rarely show vasodilation with an inflammatory infiltrate. In general, there are no histological abnormalities. The “skin tolerance threshold” is abnormally low. The skin’s barrier function is altered in some patients, leading to transepidermal water loss that may promote contact with irritants (3). The abnormal sensations and vasodilation reflect the involvement of the cutaneous nervous system (2). Neurotransmitters such as substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide may induce neurogenic inflammation with vasodilation and mast cell degranulation. In addition, non-specific inflammation may result from the release of interleukins 1 and 8, prostaglandins E2 and F2, and tumour necrosis factor α.
Although it has never been demonstrated, the role of transient receptor potential (TRP) channels in skin reactivity is obvious. Because sensitive skin is defined as a response to multiple factors that may be physical and/or chemical, an abnormal activation of TRP channels appears probable (2). In the skin, TRP channels are known to be expressed on nerve endings, Merkel cells and keratinocytes (4). TRPV1 (V: vanilloid) is activated by capsaicin, phorbol esters, heat and H +ions. TRPV3 is activated by heat and camphor. TRPV4 activation is due to heat, mechanical stresses, hypo-osmotic stress and phorbol ester derivatives. Cold and menthol activate TRPM8. TRPA1 (A: ankyrin) is activated by cold, wasabi, mustard, horseradish or bradykinin. TRP channels are probably activated by other substances that are included in cosmetic products. The activation of TRP channels is followed by Ca ++ influx into cells and then depolarisation.
Reactive skin is extremely common. In France, about 50% of individuals (59% of women and 41% of men) report having reactive skin (5), while in Japan the figures are 48% for men and 52% for women (6). This patientreported prevalence varies little across countries (5–7) and reactive skin is clearly a very frequent cosmetic problem. This prevalence increases in the summer (8), suggesting that UV exposure plays a role, but there is no association with the skin phototype (9). Although the appearance of the skin is normal in the vast majority of cases, reactive skin may occur in individuals who have another skin disorder (e.g., atopic dermatitis, seborrheic dermatitis or rosacea) (5).
Skin reactivity is not confined to the face. Among patients reporting reactive skin, 70% reported extra-facial affected sites such as the hands (58%), scalp (36%), feet (34%), neck (27%), torso (23%), or back (21%) (10). On the scalp, the semiology is somewhat different and we recently developed a new assessment scale (11, 12).
The high incidence and complex nature of sensitive skin represent a challenge for dermatologists who are faced with an increasing demand for managing this condition. The symptoms of skin sensitivity are mainly subjective and transient, so their description by subjects with sensitive skin is the best method. In order to perform studies on sensitive skin, there is a need to use standardised patient questionnaires, which allow scoring in a reproducible manner and that are suited to monitoring the evolution of the skin condition. To our knowledge, no questionnaire for measuring the severity of sensitive skin has ever been published in international literature (1, 13–15). In this work, we drew up a new questionnaire which we called the ‘Sensitive Scale’ and we studied the correlation of its scores with those of a very well-known scale on quality of life. A cream for patients with sensitive skin was used to assess the dynamic value of the Sensitive Scale.
Methods
Questionnaires
The DLQI (Dermatology Life Quality Index) is a health quality of life (QoL) scale specific to dermatological disorders (16). It comprises 10 items which focus on 6 dimensions: “Symptoms”, “Daily activities”, “Leisure”, “Work”, “Personal relationships” and “Treatment”. A total score (0–30) is calculated and can be expressed as a percentage. A higher score meant a more severe QoL impairment. The health QoL is considered impaired with a score of 6, considered very impaired with a score of 11, and extremely impaired with a score of 21 or greater (17). DLQI has been used for 33 different skin conditions in 32 countries and is available in 55 languages. Its use has been described in over 500 publications including 30 multinational studies.
The Sensitive Scale was drawn up by a group of 4 experts (3 dermatologists, 1 methodologist) who are involved in research on sensitive skin by using a standardised method (18) in 6 steps for creating and developing self-assessment questionnaires. It was initially written in French. The first step was to collect words from common language about skin sensitivity by a literature search, interviews with patients and spontaneous reporting from the 4 experts. Around 35 words were collected. In a second step, the experts eliminated synonyms and neighbouring words and identified 13 symptoms of sensitive skin: 8 subjective symptoms (tingling, burning, sensation of heat, tautness, itching, pain, general discomfort, flushes) and 5 objective symptoms (redness, scaling, swelling, oozing and scabs). A list of understandable words to describe sensitive skin was then established (third step) on the basis of these 2 groups of words. The fourth step consisted of testing this list on a panel of native French-speaking people by a cognitive debriefing to validate how the words are understood and to identify words that have to be modified to avoid misunderstandings, difficulties or ambiguity (Lionbridge, Waltham, USA). In the fifth step, 309 patients were asked to quantify each symptom from 1 to 10 on a numeric scale. A global score of skin irritability was measured from 0 to 10 by a visual analog scale. The total score ranges from 0 to 140. Finally, the sixth step consisted of translating and validating the scale in other languages (English, Chinese, Portuguese, Spanish and Italian). The English version of the 14-item Sensitive Scale (SS-14) is shown in Fig. S11. The translations were produced by Lionbridge according to its quality procedures. Cross-cultural adaptation was carried out using previously established guidelines (19, 20) in 5 stages (forward translation, a review by an expert committee, backtranslation, test/retest, psychometric validation).
Since this preliminary study showed that some symptoms (scaling, swelling, oozing and scabs) were very rarely observed in patients with sensitive skin, a shorter version of the Sensitive Scale was proposed. It consists of only 10 items and was named Sensitive Scale-10 (SS-10). The highest possible score is 100. The English version is shown in Fig. S21.
Study design
This study was performed in 11 countries: China, Colombia, Greece, Italy, Japan, Mexico, Poland, Portugal, Spain, Czech Republic and USA. Consultations were held in the main language of each country and the DLQI and Sensitive Scale were translated into these languages.
Patients with a diagnosis of sensitive skin confirmed by physicians (private practice) were included in the study. Subjects who were unable or unwilling to complete the questionnaire were excluded from the study. A dermatological exam assessing skin type, phototype and skin sensitivity was performed. The survey was anonymous and collected an assortment of information including demographics, the DLQI and the Sensitive Scale. Questions from the Sensitive Scale were given by physicians for each symptom as follows: ‘in the last 3 days, did you notice…? Could you score it from 0 to 10?’.
A second visit was arranged later in accordance with the doctor’s advice. The DLQI and the Sensitive Scale were scored. From visit 1 (V1) to visit 2 (V2), patients were assigned to apply Tolérance Extrême cream (Laboratoires Dermatologiques Avène) at least once a day on areas of sensitive skin. Tolérance Extrême cream is characterised by the use of a minimal list of 9 ingredients (Avène thermal spring water, glycerin, paraffinum liquidum, squalane, carthamus tinctorius oil, cyclomethicone, glyceryl stearate, sodium carbomer, and titanium dioxide) that excludes any kind of fragrance, colourant or emulsifier, making it particularly well-suited for sensitive skin.
Statistical analysis
All tests were two-sided. The risk of type 1 error (α) was set at 0.05 for the entire study. The programs were created using the SAS software package, version 9.2 (SAS Institute). No adjustments were made in the statistical analyses. In particular, analyses were not country-adjusted.
Qualitative variables were described in terms of numbers of subjects and percentages for the different categories. Quantitative variables were described in terms of mean, standard deviation, minimum and maximum, and median and quartiles, which constitute useful parameters where distribution exhibits marked dissymmetry. Depending on the number of values for each variable, the most appropriate description technique of ordinal variables was used (percentage of different classes or mean, median, standard deviation, minimum and maximum). Missing data items were indicated in all cases, but were omitted from the statistical tests.
Regarding qualitative variables, the missing data items were not taken into account when calculating percentages.
Moreover, statistical tests for paired-data [Student t-test or Wilcoxon signed-rank test according to the result of the previously applied normality test (Shapiro-Wilk)] were performed in order to check if the conditions of the facial skin and QoL had improved significantly during the course of the treatment from V1 to V2.
The internal consistency (21) of the Sensitive Scale was studied by computing the Cronbach α coefficient. Moreover, the correlation between the Sensitive Scale scores at V1 and the characteristics of the patients at V1 was sought by computing in the subgroup of subjects presenting sensitive skin: the Spearman coefficient of correlation for quantitative parameters and the probability associated with the variance analysis (parametric or non-parametric) for categorical parameters.
Results
A total of 2,966 patients presenting with sensitive skin were included in the study. The characteristics of these patients were as follows: (i) Age at inclusion varied from 1–89 years, with a mean age of ~39 years; (ii) women in 85.7% of the cases; (iii) skin type: dry for 34.9% of the patients, oily for 14.1% and mixed for 51.0%; (iv) phototype: I or II in 36.5% of the cases, III or IV in 61.8% and V or VI in 1.7% of the cases.
An associated dermatosis was noted in many patients: 577 (19.5%) suffered from eczema, 388 (13.1%) had seborrheic dermatitis, 377 (12.7%) acne, 270 (9.1%) rosacea and 90 (3%) another skin disease.
The cream was prescribed at V1 to be applied once daily (morning or evening) for 388 subjects (13.8%), twice a day (morning and evening) for 2,312 subjects (82.1%) and more than twice a day for 117 subjects (4.2%). On average, the physicians recommended using Tolérance Extrême for 20.1 days (SD 10.4; ranged from 7–94 days). The cream was used in monotherapy by 1,549 subjects (56.5%), associated with a topical treatment (antibiotics, antiseptics, steroids) for 932 subjects (34.0%) and in association with dermocosmetics for 260 subjects (9.5%). The conditions of use were unknown for 225 subjects (7.6%).
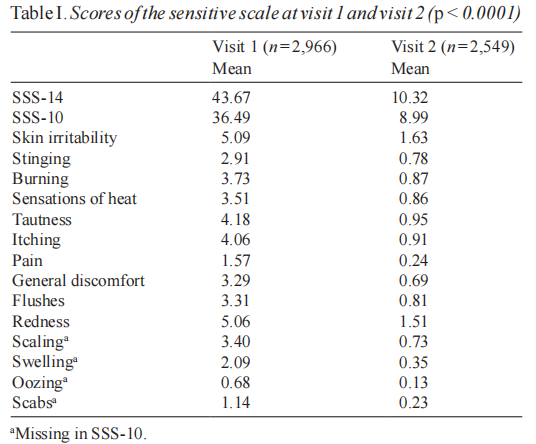
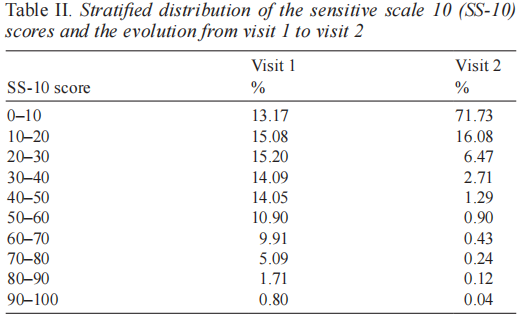
The mean Sensitive Scale score (SSS-14) decreased by 66.7% (SD 169.6) from V1 to V2 (p < 0.0001) with a mean baseline value equal to 43.7 (SD 27.9) and a mean final value equal to 10.3 (SD 14.0). The follow-up appointment was kept by 2,549 subjects. The percentage of patients whose condition improved from V1 to V2 with regards to this item was 94.4%. The mean modified Sensitive Scale score (SSS-10) decreased by 66.9% (SD 137.1) from V1 to V2 (p < 0.0001) with a mean baseline value equal to 36.5 (SD 22.3) and a mean final value equal to 9.0 (SD 11.7). The percentage of patients whose condition improved from V1 to V2 with regards to this item was 94.3%. Table I gives the detailed scores. The low pertinence of scaling, swelling, oozing and scabs was confirmed by initial scores of 0.13 to 2.69/10 for each of these criteria. Table II gives a stratified distribution of the SS-10 scores and the evolution from V1 to V2.
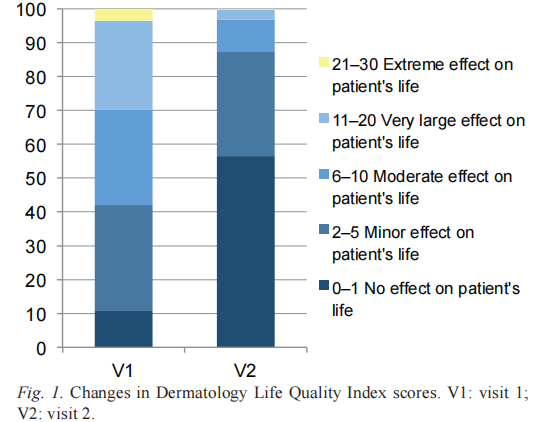
The evolution of the QoL was also analysed. The DLQI score decreased significantly showing an improvement in the QoL. In the overall population, the DLQI score decreased from a mean baseline value equal to 8.0 (SD 6.0), showing a moderate impairment of QoL, and a mean final value = 2.3 (SD 3.3), showing a significant amelioration (Fig. 1).
The internal consistency of the Sensitive Scale was good or excellent (21): Cronbach α coefficients were 0.94 for both SS-10 and SS-14. The items assessing skin irritability, burning and tautness had the best relationship with the composite scores whereas flushes had the lowest. However the α coefficient would not be improved or degraded by more than 0.02 points if any of these variables were deleted.
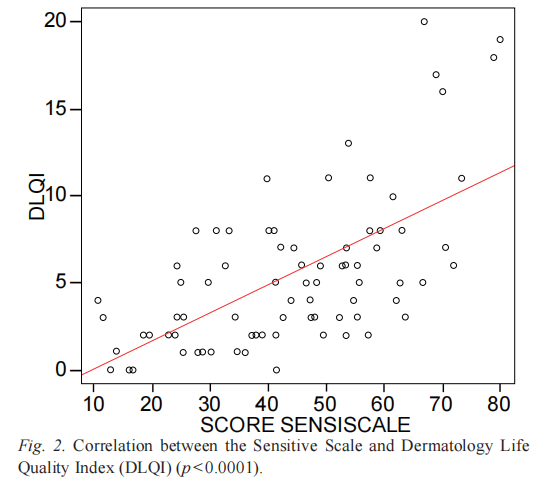
SSS-14 and SSS-10 scores at V1 were correlated with the DLQI score at V1 (r=0.58 and r=0.57, respectively; p < 0.0001) (Fig. 2). In order to identify a possible link between the Sensitive Scale scores and qualitative variables, a variance analysis was performed. They were correlated with the dry skin type, higher age, female gender and fair phototypes (p < 0.05).
Discussion
This study assesses the pertinence of the Sensitive Scale in measuring the severity of sensitive skin. The internal consistency of the Sensitive Scale was high. This scale is useful in measuring sensitive skin severity at a given time, as assessed by scores in patients with sensitive skin and the correlation with QoL. It is also well-suited to monitoring the efficacy of a treatment and has proven useful in different geographical settings. The correlation with the dry skin type, higher age, female gender and fair phototypes is an indirect confirmation of the worth of this scale because these factors are known to be associated with a higher severity of sensitive skin (1, 5–15, 22, 23).
The use of the 10-item version appears to be preferable for the following reasons: (i) it is quicker and easier to complete; (ii) the internal consistency is thev same; (iii) scaling, swelling, oozing and scabs are very rarely observed in these patients and in the literature (1, 13–15, 22).
The mean scores at V1 were 43.67/140 (SD 27.89) and 36.49/100 (SD 22.25). This suggests that people with sensitive skin could be identified with scores from 25 to 75 (or more) with SSS-14 and from 20 to 60 (or more) with SSS-10 but there is a need for further studies. In this study, individuals with SSS-14 above 75 or SSS-10 score above 60 were few: less than 10% at V1. Skin irritability and redness were the only scores over 5. Tautness, itching, burning and sensations of heat were the most frequently perceived sensations. It confirms that sensitive skin is characterised by redness (objective sign) and abnormal sensations (1, 5–10, 13–15, 22–26) and that the Sensitive Scale is a suitable tool for measuring its severity.
The strong correlation between Sensitive Scale and DLQI scores is very interesting because it enables the Sensitive Scale to be associated with a very well-known scale for measuring QoL in dermatology. It also underlines that skin sensitivity has negative effects on QoL.
The studied population was slightly different from those in previous studies on sensitive skin. In particular, a high number of patients had associated dermatosis. The explanation for that would be that in this study, patients were met in a dermatological consultation whereas they were contacted by phone in previous studies (5–9, 23). The specific patient profile of this study also reflects in the Sensitive Scale scores obtained.
The aim of this study was not to evaluate the effects of a topical treatment on sensitive skin but to assess thepertinence of a scale. To observe variations of Sensitive Scale scores over time, a cream dedicated to sensitive skin was used. However, the conditions of use were not those of a clinical trial because the time of treatment, the number of applications, the homogeneity of the population and the absence of any associated treatment were not previously defined.
The major strength of the study is the inclusion of a high number of participants with an international recruitment. A limitation of the study is the absence of re-testing to check the reproducibility and reliability of the test.
In conclusion, the Sensitive Scale is the first scale to measure the severity of skin sensitivity and then enable the measurement of variations pre- and post-treatment. The 10-item version is more convenient and better suited to the symptoms sensitive skin.
ACKNOWLEDGEMENT
Funding support: Dermatological Laboratories Avène. Conflict of interest: Laurent Misery has been consultant for A-Derma, Avène, Bioderma, Clarins, Ducray, Jonzac, Noreva, Roche-Posay and Uriage. Other authors are employees of Avène.
References
1. Berardesca E, Fluhr JW, Maibach HI. Sensitive skin syndrome. New York : Taylor & Francis, 2006.
2. Ständer S, Schneider SW, Weishaupt C, Luger TA, Misery L. Putative neuronal mechanisms of sensitive skin. Exp Dermatol 2009; 18: 417–423.
3. Branco N, Lee I, Zhai H, Maibach HI. Long-term repetitive sodium lauryl sulfate-induced irritation of the skin: an in vivo study. Contact Dermatitis 2005; 53: 278–284.
4. Boulais N, Misery L. The epidermis: a sensory tissue. Eur J Dermatol 2008; 18: 119–127.
5. Misery L, Myon E, Martin N, Verrière F, Nocera T, Taieb C. Peaux sensibles en France: approche epidemiologique. Ann Dermatol Venereol 2005; 132: 425–429.
6. Kamide R, Misery L, Perez-Cullell N, Sibaud V, Taieb C. Sensitive skin evaluation in the Japanese population. J Dermatol 2013; 40: 177–181.
7. Misery L, Boussetta S, Nocera T, Perez-Cullell N, Taieb. Sensitive skin in Europe. J Eur Acad Dermatol Venereol 2009; 23: 376–381.
8. Misery L, Myon E, Martin N, Consoli S, Boussetta S, Nocera T, et al. Sensitive skin: psychological effects and seasonal changes. J Eur Acad Dermatol Venereol 2007; 21: 620–628.
9. Misery L, Sibaud V, Merial-Keny C. Sensitive skin in the American population: prevalence, clinical data and the role of dermatologist. Int J Dermatol 2011; 50: 961–967.
10. Saint-Martory C, Roguedas-Contios AM, Sibaud V, Degouy A, Schmitt AM, Misery L. Sensitive skin is not limited to the face. Br J Dermatol 2008; 158: 130–133.
11. Misery L, Sibaud V, Ambronati M, Macy G, Boussetta S, Taieb C. Sensitive scalp: does this condition exist? An epidemiological study. Contact Dermatitis 2008; 58: 234–238.
12. Misery L, Rahhali N, Ambonati M, Black D, Saint-Martory C, Schmitt AM, et al. Evaluation of sensitive scalp severity and symptomatology by using a new score. J Eur Acad Dermatol Venereol 2011; 25: 1295–1298.
13. Slodownik D, Williams J, Lee A, Tate B, Nixon R. Controversies regarding the sensitive skin syndrome. Expert Rev Dermatol 2007; 2: 579–584.
14. Farage MA, Maibach HI. Sensitive skin: closing in on a physiological cause. Contact Dermatitis 2010; 62: 137–149.
15. Diogo L, Papoila AL. Is it possible to characterize objectively sensitive skin? Skin Res Technol 2010; 16: 30–37.
16. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216.
17. Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J Invest Dermatol 2005; 125: 659–664.
18. Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a multiple ability self-report questionnaire. J Clin Exp Neuropsychol 1994; 16: 93–104.
19. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993; 46: 1417–1432.
20. Misery L, Schmitt AM, Boussetta S, Rahhali N, Taieb C. Melasma: measure of the impact on quality of life using the French version of MELASQOL after cross-cultural adaptation. Acta Derm Venereol 2010; 90: 331–332.
21. Kline, P. The handbook of psychological testing (2nd ed.). London: Routledge, 1999.
22. Farage MA, Katsarou A, Maibach HI. Sensory, clinical and physiological factors in sensitive skin: a review. Contact Dermatitis 2006; 55: 1–14.
23. Jourdain R, de Lacharrière O, Bastien P, Maibach HI. Ethnic variations in self-perceived sensitive skin : epidemiological survey. Contact Dermatitis 2002; 46: 162–169.
24. Marriott M, Whittle E, Basketer DA. Facial variations in sensory responses. Contact Dermatitis 2003; 49: 227–231.
25. Farage MA. Perceptions of sensitive skin: changes in perceived severity and associations with environmental causes. Contact Dermatitis 2008; 59: 226–232.
26. Farage MA. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clin Exp Dermatol 2009; 34: e521–e530.
This article is excerpted from the Acta Derm Venereol 2014; 94: 635–639 by Wound World.


