The lifetime risk of developing a foot ulcer is between 15–25% among patients with diabetes mellitus (Singh et al, 2005). These ulcers lower the quality of life for the affected patients (and their families), require time-consuming medical care, generate great financial cost and are associated with substantial morbidity and increased mortality. Complications related to diabetic foot ulcers (DFUs) are now the main cause of diabetesrelated hospitalisations and of non-traumatic lowerextremity amputations globally. Thus, it is necessary to treat DFUs as quickly and effectively as possible.
When the DFU has not yet penetrated to deep subcutaneous tissues and infection is either absent or under control, clinicians can treat the wound with secondary healing. If the ulcer is deep, involving subcutaneous fat, ligaments or bone, treatment usually requires surgical procedures. Wellgranulated wounds with proper wound care can be skin grafted; for reconstruction of deep, complex wounds, local, distant or free-flap operations are mandatory (Jeon et al, 2013). Although STSG application is a relatively simple surgical technique, in wound reconstruction several factors, including in adequate wound bed preparation, may cause partial or total graft loss (Rose et al, 2014). Thus, it is essential to ensure the wound is uninfected and has healthy granulation tissue before performing surgery. Many surgical and nonsurgical techniques have been described to enhance appropriate granulation tissue, including serial debridement, negative pressure wound therapy, platelet-rich plasma, hyperbaric oxygen therapy and administration of growth factor administrations (Yeh et al, 2010; Berlanga-Acosta et al, 2013). In chronic DFUs, in addition to peripheral vascular disease and distal symmetric polyneuropathy, the healing process is impaired in part by a deficiency of growth factors (Loot et al, 2002). These factors, which are poorly secreted or degraded in a chronic wound due to biofilm or infection, are also essential in the formation of granulation tissue (Pouget et al, 2020). Epidermal growth factor (EGF) has been shown to have a critical role in angiogenesis and formation of healthy granulation tissue in DFUs (Berlanga-Acosta et al, 2013).
EGF is a 53-aminoacid polypeptide isolated from adult mouse submaxillary glands that exerts potentmitogenic activity through binding to a specific cell membrane receptor (Cohen, 1962; Tsang et al, 2003). EGF was first discovered in 1962 and intralesional recombinant EGF form was first produced and licensed in the Center for Genetic Engineering and Biotechnology (Havana, Cuba) in 2006 as an injection for treating patients with a diabetic foot wound, as an adjunct to standard treatment to accelerate wound healing. Intralesional EGF is a newly registered medication in Turkey, approved for treat of diabetic foot wounds. In this study, we present our preliminary observations of the effects of treatment with intralesional EGF on STSG survival in patients with a DFU.
Materials and methods
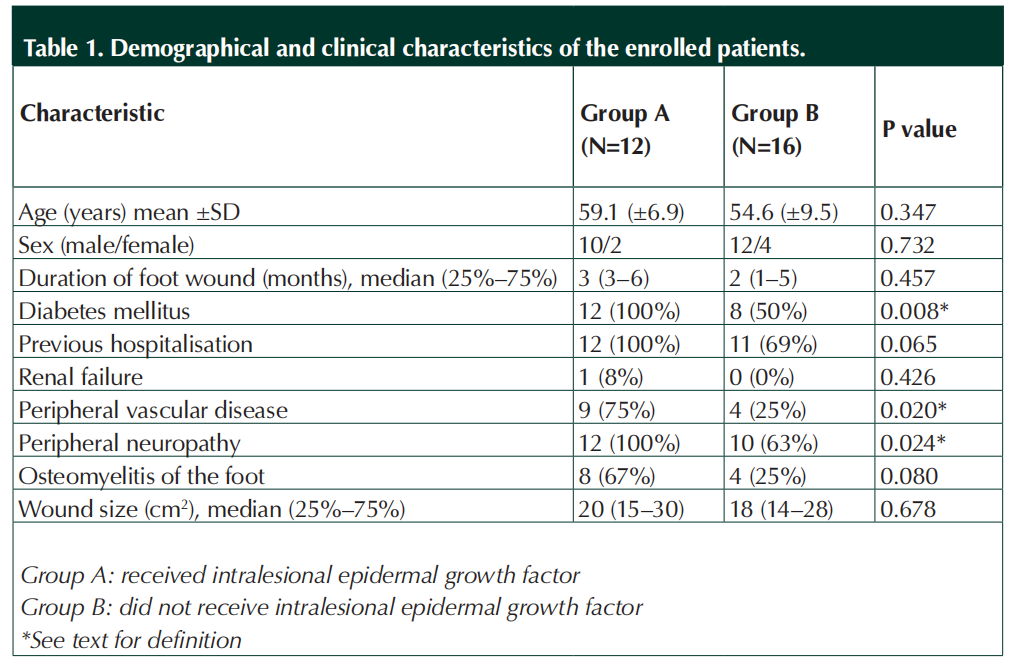
This retrospective study was designed to review patients treated at our medical centre for DFUs from January 2012 (when intralesional EGF was first licensed in Turkey) to August 2018. We included in this study all patients who visited our clinic for a chronic foot ulcer due to diabetes or other causes (diabetic and nondiabetic) who underwent reconstructive surgery with an autologous skin graft, whether or not they received intralesional EGF. Among 28 available patients who visited our clinic and had standard wound care, 12 diabetic patients also received intralesional EGF prior to application of skin grafts (designated as group A), while the remaining 16 patients who underwent autologous STSG after standard wound care for reconstruction, but without intralesional EGF (designated as group B). Eight patients (50%) were diabetic in group B. Patients with foot wounds in group A had a higher risk of vascular impairment (p=0.02) and neuropathy (p=0.024) than the patients with foot wounds in group B. From the detailed forms routinely used in the follow-up of DFU patients in our hospital’s chronic wound care unit, we collected pertinent demographic and clinical patient data, including, age, sex, previous hospitalisations and presence of lower extremity osteomyelitis, neuropathy, or peripheral vascular disease. A trained physician examined each patient’s foot and assessed for pathology using the International Working Group on the Diabetic Foot’s PEDIS classification (perfusion, extend/size, depth/ tissue lost, infection, sensation; Lipsky et al, 2012).
Although physical examination was routinely performed, all patients also underwent either magnetic resonance angiography or Doppler ultrasonographies. The majority of the patients also had endovascular procedures, but none had bypass surgery. We diagnosed foot osteomyelitis by positive results on any one of the following tests: bone biopsy for culture or histopathology, X-ray, or magnetic resonance imaging (MRI). Finally, we diagnosed peripheral neuropathy by abnormal findings on the monofilament test result or other diagnostic tests conducted by a neurologist. All patients included in this study were admitted to our hospital. On each patient’s admission, clinicians obtained specimens of the DFU for culture by tissue curettage, needle aspiration or biopsy (depending on the wound depth), following wound cleansing and debridement.
Intralesional EGF therapy
Patients in group A received intralesional injections of 75µg EGF (Heberprot-P®, Cuba) three times per week on alternate days. Application sites were prepared with debridement of necrotic, infected soft tissues and bone (in patients with osteomyelitis) and concurrent antibiotic therapy. Following control of infection, EGF was administered by the infectious diseases physician. In all cases, intralesional EGF treatment was only initiated following infection control measures, but it was not required to wait for completion of the course of antibiotic therapy. Thus, antibiotic therapy and EGF injections were both continued during the treatment period. Patients were discharged from the hospital when they were clinically stable and they then received intralesional EGF on an outpatient basis. All intralesional EGF injections were performed in hospital settings but the patients were discharged and the injections were performed in the outpatient part of our chronic wound care unit.
For outpatient treatment, the EGF vials were transferred to the treating centre as lyophilised form with cold chain procedures and stored at 4–8C°. EGF was dissolved in 5ml of sterile water for injection. In each application, this volume was distributed throughout the lesion, in 0·5– 1ml injections, starting from the deeper zones (Fernandez-Montequin et al, 2009). In the outpatient clinic, EGF was injected in the wound by the infectious disease specialists.
Autologous STSG application
After the wound showed sufficient granulation tissue, EGF therapy was terminated and the STSG operation was performed as soon as possible. The same criteria were also used by treating clinicians to select the timing of grafting in the non-EGF treated patients (group B). All the STSGs were of the same thickness, obtained with the same type of electric dermatome from the patient’s lateral thigh region. Treating clinicians used skin staplers and negative pressure wound therapy to stabilise grafts. The negative pressure wound dressings over the grafts were routinely opened on day five and covered daily with Bactigras® (Smith & Nephew, Canada). The graft donor sides were closed with Kaltostat and the dressing was opened two weeks postoperatively. Viabilities of the grafts were assessed macroscopically after 8 weeks by percentage: ≤25% (no viability, Grade 0), 26–50% (minimal viability, Grade 1), 51–75% (partial viability, Grade 2) and >76% or wound closure (complete viability, Grade 3).
Inclusion criteria
1. Patients who were hospitalised for a foot wound
2. Patients who underwent autologous STSG.
Statistical analysis
For this study we used proportional comparisons for categorical variables to calculate chi-square values and the Kolmogorov-Smirnov test to determine if there was a normal distribution of constant variables. We used Student’s t-test for variables with a normal distribution and the nonparametric Mann–Whitney U Test for variables without a normal distribution and set the level of statistical significance as a p value of <0.05.
Results
We found a total of 28 patients who had foot ulcers at various sites who met our inclusion criteria for this retrospective study. Twelve patients had received intralesional EGF in addition to standard wound care prior to their STSG (group A), while16 patients had their STSG after only standard wound care (group B). As shown in Table 1, there were no statistically significant differences between the groups in their mean age, sex, duration of the foot wound, wound size or presence of renal failure. However, patients in group A had a significantly higher rate of peripheral vascular disease and neuropathy. Treatment was for a DFU in all 12 patients in group A, but for only eight (50%) in group B (p=0.008). Among the other patients in group B, six (37%) were treated because of a chronic wound following trauma and two (13%) for a neuropathic ulcer.
All of the patients in group A had been hospitalised elsewhere for various durations in several different medical centres for management of the same wound (whether infected or not). In each case the treating clinicians elsewhere had recommended an amputation at various locations below the knee. Using the PEDIS infection classification, nine (75%) of the group A patients were Grade 3, while the rest were Grade 4. For each of these patients, clinicians sharply debrided wound callus, fibrin and necrotic material and washed them with saline solution. Osteomyelitis underlying the DFU was diagnosed in eight group A patients (67%) and four (25%) group B patients. Clinicians performed minor amputations (trans-metatarsal or toe) on 10 patients in group A and four in group B. No patient in either group underwent a major amputation.
All patients received appropriate systemic antibiotics according to the wound culture and susceptibility results as well as other standard wound care procedures and diabetes associated medical treatment. Infection was clinically controlled in all patients both groups. Group A patients received a total of 152 intralesional EGF injections by the end of the study. No patient in either group experienced a donor site complication. Complete graft survival occurred in six (50%) of the patients in group A and four (25%) in group B (p=0.04). Patients in group A had significantly fewer post-operative complications (17% versus 75%, p=0.008) and shorter duration of hospitalisation (5.8 versus 10.9, p<0.001) than those in group B (Table 2). Minimum follow-up period was three weeks after graft healing for all patients.
Discussion
STSG is a well-known and widely accepted method for soft tissue coverage of open wounds (Chick, 1988). This technique plays a major role in reconstructing burn wounds and other skin defects. While STSG has also been used successfully to treat chronic DFU, it has rarely been discussed as a primary means of diabetic foot reconstruction (Roukis and Zgonis, 2005). Adequate wound bed preparation before application of skin grafts is critical to operative success. When wounds are uninfected and have healthy granulation tissue, progress towards epithelialisation generally occurs. Skin grafts promote wound healing and reduce the time needed for wound healing (Robson et al, 1990). However, diabetic patients have defective wound healing largely related to vascular and neurological disorders, but also in part due to deficiencies of growth factors (Loot et al, 2002). Glucose binds proteins non-enzymatically and, by a series of reactions, this leads to advanced glycosylation end products (AGE) in long-term hyperglycaemic patients. These end products bind macrophages and induce secretion of various cytokines, mainly TNF-α, and by increasing reactive oxygen species (ROS) cause low-grade inflammation (Berlanga-Acosta et al, 2013). In a DFU, this condition causes apoptosis of endothelial cells and fibroblasts by inducing mitochondrial damage and also decreases production of ECM, growth factors and receptors for these growth factors. Ultimately, the above-mentioned factors result in decreased formation of granulation tissue (Acosta et al, 2008).
EGF has both mitogenic and motogenic roles as well as cytoprotective actions in wound healing. It stimulates: (i) the migration of productive cells to the ulcer area; (ii) formation of granulation tissue, including extracellular matrix accumulation, maturation and de novo angiogenesis; (iii) wound contraction by myofibroblast activation and proliferation; and (iv) resurfacing of damaged areas by epithelial cell migration and proliferation (Berlanga-Acosta, 2011). EGF plays a dominant early role in wound healing by stimulating keratinocyte proliferation and migration (Gibbs et al, 2000). In the selection and timing of skin graft reconstruction, surgeons macroscopically assess the base of the wound, with healthy granulation tissue defined as being odourless, bleeding easily and bright red coloured. The angiogenetic potential of the granulation tissue plays an essential role in successful wound healing. As EGF stimulates the angiogenetic potential of the wound and increases both the amount and quality of granulation tissue formation, the granulation tissue generated by its application may be angiogenetically superior, promoting graft take. To further assess the effect of EGF, it would be helpful to conduct a study that obtained biopsies from the granulation tissues comparing wounds to which EGF was those to which it was not.
In our study, patients in group A had demographic and clinical characteristics that would likely put them at higher risk of graft failure thanthose in group B. Peripheral arterial insufficiency and peripheral neuropathy have been shown to be risk factors for graft failure (Krishnan et al, 2007),and these were significantly more frequent in group A patients. These group A patients who received intralesional EGF had a complete graft viability rate (Grade 3) of 50%, compared to 25% in group B.
In our study, graft healing rates were not significantly different in the two treatment groups (75% and 69% in group A and group B, respectively). In a review of 83 diabetic patients who underwent STSG placement for foot wounds, Ramanujam et al found that 65% experienced initial graft take within seven weeks postoperatively (Ramanujam et al, 2010). Rose et al (2014) reported a similar rate of 66.7% for diabetic patients in their study. Likewise, Yeh et al (2010) reported a graft take rate of 74% in the first month of evaluation and 86.3% in the second month of evaluation. The study of diabetic foot patients by Mahmoud et al (2008), however, reported a high total graft loss of 38%. It is noteworthy that none of these studies had information about the peripheral vascular or neuropathic status of their patients. All of our group A patients were hospitalised in other centres and offered major lower extremity amputations. Thus, these patients had advanced stages of DFU, but none required major amputation and none had a complete graft loss. This is despite the fact that our patients had a relatively high proportion with peripheral vascular disease (75% and 25% in groups A and B, respectively) and peripheral neuropathy (100% and 63% in groups A and B, respectively). These diabetes-related foot complications generally impair wound and graft healing, but the rate of graft healing was significantly higher in the intralesional EGF group (group A).
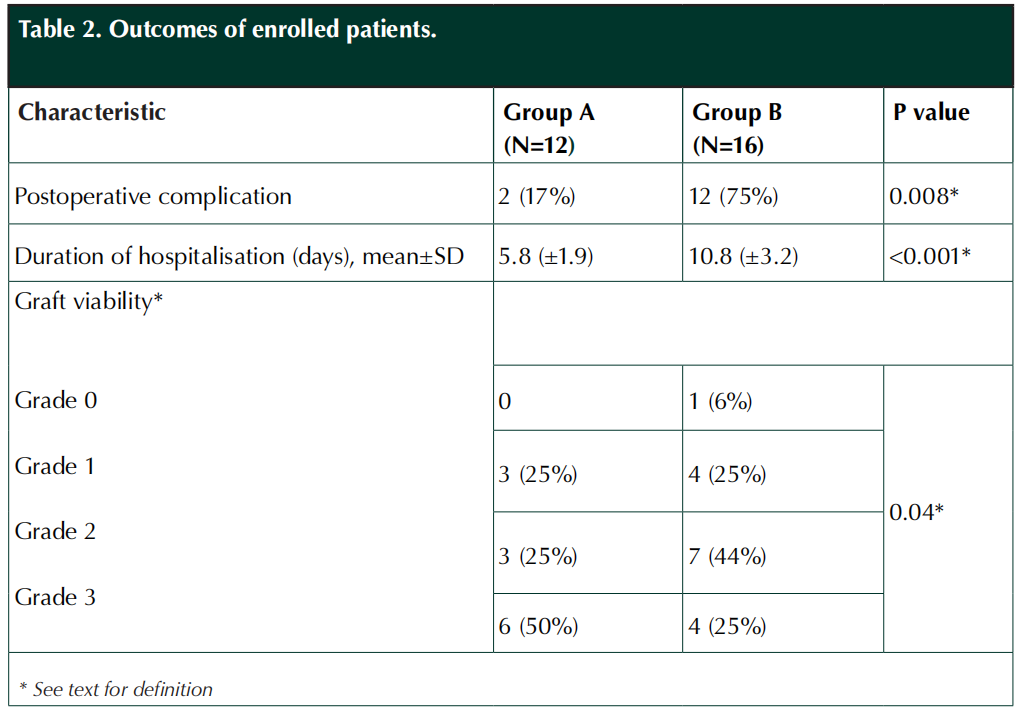
Postoperative complications are another important problem for patients undergoing STSG, with reported rates ranging from 2.6% and 35% in different studies (Mahmoud et al, 2008; Ramanujam et al, 2010; Anderson et al, 2012). Complication rates have been related to both postoperative care and preoperative wound preparation, as well as various external factors. Therefore it may be difficult to compare complication rates between different populations. In our study, the postoperative complication rates were significantly lower in group A than group B patients (17% and 75%, respectively) despite the fact that the groups had similar treatment in the same hospital during the postoperative period. Similarly, the duration of hospitalisation was significantly shorter for the EGF-treated group A patients (mean±SD; 5.8±1.9 days) than it was for the group B patients who only received the standard wound care (mean±SD; 10.8±3.2 days). These findings suggest that intralesional EGF treatment may be associated with the beneficial effect of a reduced rate of both postoperative complications and a shortened hospital stay, but this would need to be confirmed in a larger, prospective trial.
This study has several important limitations. First, we were only able to report on a relatively small number of patients. Second, the study was a retrospective review of patients seen in our medical centre. We do not have data on potentially important parameters such as wound granulation, angiogenesis and fibroblast proliferation in patients who did not receive intralesional EGF. This is, however, one of the few studies of treatment with this new therapy in patients with DFU.
Conclusion
In this small, retrospective study, treatment of patients with (mostly diabetic) foot wounds with intralesional EGF before an STSG operation was associated with an increase in graft viability, a reduction in postoperative complication rates and a shorter duration of hospitalisation. These results suggest it would be useful to attempt to verify these results in prospective studies with a larger population.
Acknowledgments
Professor Benjamin A Lipsky, Professor of Medicine Emeritus at University of Washington, US, and Visiting Professor of Medicine, University of Oxford, UK, for his critical review of the manuscript and enlightening advice.
Conflict of interest
No conflicts of interest.
References
1. Acosta JB, del Barco DG, Vera DC et al (2008) The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J 5: 530–9.
2. Anderson JJ, Wallin KJ, Spencer L (2012) Split thickness skin grafts for the treatment of non-healing foot and leg ulcers in patients with diabetes: a retrospective review. Diabet Foot Ankle 3: doi: 10.3402/dfa.v3i0.10204
3. Berlanga-Acosta J (2011) Diabetic lower extremity wounds: the rationale for growth factors-based infiltration treatment. Int Wound J 8: 612–20
4. Berlanga-Acosta J, Mendoza-Mari Y, Martinez MD, et al (2013) Expression of cell proliferation cycle negative regulators in fibroblasts of an ischemic diabetic foot ulcer. A clinical case report. Int Wound J 10: 232–6
5. Chick LR (1988) Brief history and biology of skin grafting. Ann Plast Surg 21: 358–65
6. Cohen S (1962) Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem 237: 1555–62
7. Fernandez-Montequin JI, Betancourt BY, Leyva-Gonzalez G et al (2009) Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: treatment up to complete wound closure. Int Wound J 6: 67–72
8. Gibbs S, Silva Pinto AN, Murli S et al (2000) Epidermal growth factor and keratinocyte growth factor differentially regulate epidermal migration, growth, and differentiation. Wound Repair Regen 8: 192–203
9. Jeon H, Kim J, Yeo H et al (2013) Treatment of diabetic foot ulcer using matriderm in comparison with a skin graft. Arch Plast Surg 40: 403–8
10. Krishnan, ST, Quattrini C, Jeziorska M et al (2007) Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care 30: 3058–62
11. Lipsky BA, Berendt AR, Cornia PB et al (2012) 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 54: e132–73
12. Loot MA, Kenter SB, Au FL et al (2002) Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol 81: 153–60
13. Mahmoud SM, Mohamed AA, Mahdi SE, Ahmed ME (2008) Split-skin graft in the management of diabetic foot ulcers. J Wound Care 17: 303–6
14. Pouget C, Dunyach-Remy C, Pantel A et al (2020) Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 8(10): 1580
15. Ramanujam CL, Stapleton JJ, Kilpadi KL et al (2010) Split-thickness skin grafts for closure of diabetic foot and ankle wounds: a retrospective review of 83 patients. Foot Ankle Spec 3: 231–40
16. Robson MC, Stenberg BD, Heggers JP (1990) Wound healing alterations caused by infection. Clin Plast Surg 17: 485–92
17. Rose J, Giovinco N, Mills JL et al (2014) Split-thickness skin grafting the high-risk diabetic foot. J Vasc Surg 59: 1657–63
18. Roukis TS, Zgonis T (2005) Skin grafting techniques for soft-tissue coverage of diabetic foot and ankle wounds. J Wound Care 14: 173–6
19. Singh N, Armstrong DG, Lipsky BA (2005) Preventing foot ulcers in patients with diabetes. JAMA 293: 217–28
20. Tsang MW, Wong WK, Hung CS et al (2003) Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 26: 1856–61
21. Yeh JT, Lin CH, Lin YT (2010) Skin grafting as a salvage procedure in diabetic foot reconstruction to avoid major limb amputation. Chang Gung Med J 33: 389–96
This article is excerpted from the 《The Diabetic Foot Journal Vol 27 No 1 2024》 by Wound World.


