According to the CDC, surgical site infection (SSI) is defined as an infection related to a surgical procedure that occurs near the surgical site within 30 days after surgery (or up to 90 days after surgery when it comes to an implant). The overall incidence of infection in heart transplantation is 30-60%, with a mortality rate of 14-15%. The most frequent infections in these patients are bacterial (43 to 60%) followed by viral (40 to 45%) and fungal and those caused by protozoa (8 to 14%).
The aim of this case report is to describe the management and care of surgical site infection by Gram-negative bacillus after a heart transplant, infection being one of the consequences of immunosuppression, patient flora and the antibiotics administered within the first month prior to transplantation, guided by culture of secretion. Closure therapy was used, with a transparent cellulose membrane and antiseptic broad-spectrum germicidal with hypochlorous acid content, allowing 100% epithelialisation of wound.
Risk factors
Risk factors for heart transplant patients include neutropenia, immunosuppression, diabetes mellitus, obesity, malnutrition, prior cardiac procedure, prolonged mechanical ventilation, prior ventricular assist device and transplant reoperation.
Physiopathology
Microbial contamination of the surgical site is a necessary precursor to infection. The risk of infection can be conceptualised quantitatively: if a surgical site is contaminated with more than 105 microorganisms per gram of tissue, the risk of SSI increases considerably. However, the dose of contaminating microorganisms needed to produce infection may be much lower when foreign material is present at the site (i.e. 100 staphylococci per gram of tissue introduced into the silk sutures). This may cause a delay in healing.
Surgical procedure exposes sterile body tissues, where microorganisms introduced during the procedure can multiply into the wound after it has closed and subsequently cause infection of the surgical site. Moreover, accurate diagnosis of infection is difficult to undertake as it may take several weeks to develop and infection may not be evident.
Microbiology
In heart transplant patients, the microorganisms most commonly found in surgical site infections are: coagulase-negative Staphylococcus spp; S. aureus, including methicillin-resistant Staphylococcus aureus (MRSA); Enterococcus; Enterobacteriaceae, including extended-spectrum beta-lactamase organisms (ESBL); Pseudomonas aeruginosa; Stenotrophomonas; and Candida species. Escherichia coli, Klebsiella pneumoniae and P Pseudomonas aeruginosa Iare the most frequently isolated microorganisms.
Klebsiella spp. can be found in the environment and can be asymptomatic; it is commonly acquired in intensive care settings. It is frequently found in wound infections, intravascular device infections and central nervous system infections. But it has been seen in immunosuppressed patients who are more susceptible to klebsiella spp. infection.
Zhou et al (2021) reported that the bacteria most commonly found in heart transplant wounds are Klebsiella Pneumoniae with 18.9% and Acinetobacter baumanii with 8.1%.
Diagnosis
When possible, an interdisciplinary team of specialists should perform the evaluation and diagnosis of a possible SSI. These wound teams are usually composed of doctors, nurse practitioners, surgeons, infectologists, pharmacists, prosthetists/orthotists, and others. The use of diagnostic tests can provide the physician with the appropriate level of information to allow for an informed diagnosis, along with clinical evaluation. SSI can affect the superficial cutaneous layer. Local infections may present certain manifestations of signs and symptoms such as erythema, local warmth, edema, purulent discharge, wound breakdown and enlargement, new or increasing pain and increasing malodour.
Tissue and fluid analysis allows early identification of pathogenic activity and determination of appropriate management regimens.
Blood test results show high C-reactive protein content, erythrocyte sedimentation rate, high procalcitonin, high leukocyte cells, and systemic inflammatory response.
Wound dehiscence can be caused by issues other than microorganisms, such as obesity or pre-existing chronic diseases.
When clinical signs and symptoms of infection are present, wound cultures are useful to indicate the pathogen. These cultures can be gathered via the aspiration of tissue or fluid.
Treatment
There are currently very few studies regarding SSIs in cardiac transplant recipients, and most of them describe epidemiology and risk factors. Yet, so far, no recommendation on treatment has been published. Therefore, most of the following recommendations are for the non transplanted population.
All clearly infected wounds should be irrigated, debrided, and treated using basic wound care procedures. However, wound swab cultures often reveal polymicrobial growth, making it difficult to distinguish colonisation from a true infection. Ideally, cultures should be gathered using aspiration or sterile tissues, but swabs are useful if careful interpretation of the results is used. Culture results should be used to guide the choice of antibiotics, particularly if the patient is at high risk due to resistant pathogens. Superficial SSIs can be treated by opening the wound enough for proper visualisation of the underlying tissue to ensure that no further processes occur.
Infected incision wounds should be irrigated with an isotonic solution, such as a saline solution, to remove loose dead tissue and exudate, and mechanical debridement with shear excision or pressure irrigation may be indicated to remove devitalised tissue. Once the wound has stabilised and most of the devitalised tissue has been removed, antiseptic, antimicrobial, or a surfactant solution should be used to promote cleaning of the peri-lesional surface and skin and dressing or cell membrane placement applied to remove residual biofilm, preventing potential contamination and recolonisation. The need for antibiotic therapy should be determined by the extent of infection, evidence of systemic involvement, and patient status, including comorbidities. While uncomplicated superficial SSIs that have been treated with incision and drainage can generally be treated without antibiotics, in immunocompromised individuals, targeted antimicrobial therapy is recommended in solid organ transplant recipients to prevent spread.
Case presentation
A 27-year-old patient with a history of reduced LVEF chronic heart failure (21%) NYHA III, diagnosed in November 2021 secondary with hamartoma of mature cardiac myocytes, with bicaval orthotopic heart transplantation on July 17, 2022, using prophylactic antibiotics following surgery with trimethoprim (160mg) and sulfamethoxazole (800 mg) once daily for three days.
Subsequently, the patient was readmitted on September 2, 2022, to be evaluated by the medical team, who found a surgical wound with a visible tattoo presenting exposed subcutaneous layer without visible fascia, oedema, thick and purulent secretion outflow in moderate quantity of the incision, increase of pain in the area of surgery with an EVA of 3 on palpation and separation of the edges in soft tissue. It was therefore decided to take bone scan, blood biometrics and a culture of the secretion present, using aspiration and having disinfected the skin with 10% povidone iodine and having left this to dry for 1 minute. Thereafter, the puncture-aspiration of the wound was performed, infiltrating 1ml of 0.9% saline solution and aspirating in the area with the most infected tissue. Finally, antibiotic treatment, using Ertapenem (1g) was commenced, prior to the culture result [Figure 1].
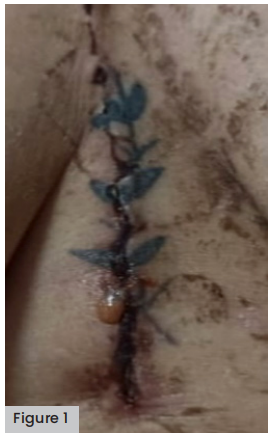
Figure 1. Sternal wound with visible tattoo presenting thick, purulent discharge, in moderate quantity, and pain
On September 5, 2022, a growth of bacteria in culture positive to Klebsiella was detected, and blood biometrics showed leukocytosis and a negative reading in the bone scan, having maintained the Ertapenem 1g for 3 weeks intravenously. The patient was assessed by the hospital wound team and the wound was cleaned with broad-spectrum antiseptic and germicidal with hypochlorous acid content, leaving it to act for 1 minute on the wound, before performing an acute debridement with a scalpel, resulting in a 4cm wound with 100% slough tissue. Again, wound and wound edge cleaning was performed with the same antiseptic before a transparent, porous, cellulose membrane (Membracel®) measuring 7.5 x 5cm of medium porosity was placed 1cm outside the wound bed for better adhesion. A thin hydrocolloid dressing to keep hydrated and avoid the need for removal during the hospital stay, was also placed over the wound.
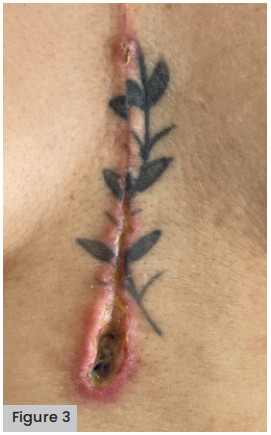
Figure 2. Preparation of the wound bed, finding 100% slough. Porous cellulose membrane is placed.
On September 7, 2022, the thin hydrocolloid dressing is removed, finding a wound with a decrease in slough tissue with a depth of 0.5mm, the wound was cleaned with antiseptic and cellulose membrane was again placed, leaving it wet with the antiseptic; this was covered with sterile gauze to be assessed and hydrated every day. [Figure 3].
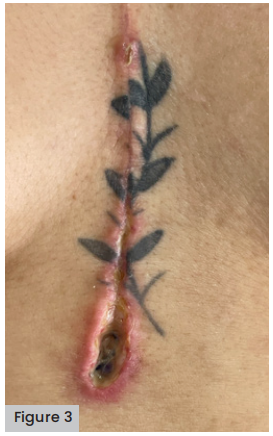
Figure 3. Two days after changing the membrane, it is possible to see the decrease in the slough and depth of the wound
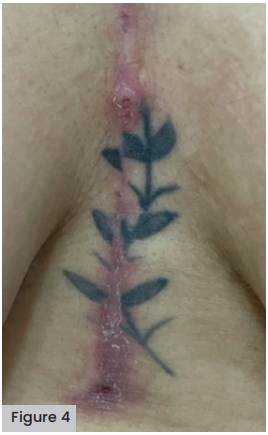
The same procedure was performed for 30 days until 100% epithelialised tissue was obtained. [Figure 4].
Discussion
The aim of treating infected wounds is to minimise infection with appropriate antibiotic therapy, proper wound cleaning with antiseptic, and the use of antimicrobial dressing or cell membrane if necessary, while encouraging the development of healthy granulation tissue and initiating the process of healing.
In accordance with localised epidemiology results, the type of transplant procedure carried out, the colonisation status, the risk factors for difficult-to-treat pathogens, the clinical severity of the infection, the source of the infection and recent infections, empirical treatment should be initiated with broad spectrum antibiotics covering the gram positive cocci of the skin, as well as the expected flora at the site of the operation
As for wound hygiene, it is always important to eliminate obstacles so that the antimicrobial dressing or membrane that is placed acts with maximum efficacy. Antiseptics used in antimicrobial dressings or membranes are of great importance in wound hygiene, as they break down biofilm, eliminate biofilm organisms, and prevent reformation or colonisation. However, this does not mean that wound hygiene should be applied in a standard way in all cases, since not all wounds require the same topical dressing treatments or membranes. Moreover, the scientific basis of wet environment therapy has been gradually accepted by researchers since the lack of water in wounds affects cell metabolism and leads to the drying of cells. In addition, the moist environment inside a wound not only promotes tissue regeneration, but also relieves pain.
Transparent porous cellulose membrane has been used since the 1980s and has been widely accepted in wound treatment, as it is a natural polysaccharide produced by culturing the bacterium Komagataeibacter xylinus.
Due to its unique properties, such as its three-dimensional nonwoven network, its ability to transport large volumes of liquid, its high tensile strength in the wet state and its excellent permeability and biocompatibility, transparent porous cellulose membrane has considerable potential as a biomaterial for tissue engineering applications, especially for the repair of skin lesions (Naomi et al, 2020).
A variety of studies have shown that, compared to wound dressings that are already on the market, such as chitosan and alginate, transparent porous cellulose membrane offers superior water retention, biocompatibility, mechanical strength and does not adhere to wounds.
Jain et al (2005) mention that cellulose membranes were able to restore injured tissue more effectively than gauze. This can be attributed to the 3D micropore structure of the membranes, because the appropriate pore size present within the 3D scaffolds supported the migration of cells and the transport of nutrients, waste and oxygen.
Although the membrane itself lacks antibacterial properties (Wei et al, 2011), several studies exist in which the membrane has been modified with silver nanoparticles to reduce wound infection, generating positive results. Furthermore, the choice of strain is important for healing.
Cellulose membrane has been used in multiple pathologies, such as burns, where Vieira et al (2007) analysed the cellulose membrane in 29 patients with second-degree, superficial, and deep burns with an average of 12% of the body surface burned. Decontaminating the wound with 4% chlorhexidine gluconate was carried out for seven days for superficial and epithelialised superficial burns and 12 days for deep burns. In another study (Cohelo et al, 2020), the same membrane was used for second-degree burns in rats, and an absence of pain in membrane replacement and an advanced state of wound healing until day 20 were observed, as well as a higher proportion of mature collagen in burns.
Finally, in a 2019 randomised, controlled clinical trial, the cellulose membrane was placed in venous ulcers with considerable decrease in pain noted after 4 weeks.
This case report describes the use of the Komagataeibacter xylinus strain cellulose membrane only, accompanied by the use of broad-spectrum antiseptic and germicidal treatment with hypochlorous acid content in an infected wound in a postoperative heart patient, allowing the closure of the wound without complications and without having to use adjuvant therapies or reoperation over a period of 30 days.
Patients who undergo heart transplants are at a high risk of SSI, so reducing risk factors will help reduce future complications and extended hospital stays. This paper presents the limitations of any case report.
Conclusions
SSIs in post-transplant patients account for the majority of infections in the immediate post-transplant period. This is, in part, due to the complexity of these operations, immunosuppression and patient comorbidities, which should be diagnosed clinically and treated early with the help of cultures, antibiotics, antiseptics and dressings or membranes according to the wound.
Ethical considerations
The patient voluntarily authorised the disclosure of the information presented in this case study.
References
1. Abbo MI, Grossi PA (2019) Surgical site infections: guidelines from the american society of transplantation infectious diseases community of practice. Clin Tran
2. Atiyeh BS, Dibo SA, Hayek SN (2009) Wound cleansing, topical antiseptics and wound healing. Int Wound J 6(6): 420–30
3. Coelho GA, Magalhães MAB, Matioski A et al (2020) Pine nanocellulose and bacterial nanocellulose dressings are similar in the treatment of second-degree burn? Experimental study in rats. Arquivos Brasileiros de Cirurgia Digestiva 33(2)
4. Gurguí MY, Muñoz P (2007) Infecciones en el trasplante cardíaco. Enfermedades Infecciosas y Microbiología Clínica 25(9): 587–98
5. Horan TC, Andrus MY, Dudeck MA (2008) CDC/NHS surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 36(5): 309–32
6. Ioannou P, Miliara E, Baliou SY, Kofteridis DP (2021) Infective endocarditis by klebsiella species: a systematic review. Journal of Chemotherapy 1–10
7. Murphy C, Atkin L, Swanson T et al (2020). Defying hard to-heal wounds with an early antibiofilm intervention strategy: wound hygiene. Journal of Wound Care 29(sup3b): s1—26
8. Nunes CADB, Melo PG, Malaquias SG et al (2019) Effectiveness of two bundles in venous leg ulcer healing: a randomized controlled trial. Journal of Vascular Nursing 37(4), 232–45
9. Stryja J, Sandy-Hodgetts K, Collier M et al (2020) Prevention and management across health-care sectors. Journal of Wound Care 29(sup2b): s1—72
10. Yaguishita N (2007) Avaliação da cicatrização induzida pela membrana de celulose porosa depois da retirada total da pele em dorso de ratos. Jornal Vascular Brasileiro 6(2), 193–94
11. Yuan H, Chen LY, Hong FF (2022) Evaluation of wet nanocellulose membranes produced by different bacterial strains for healing full-thickness skin defects. Carbohydrate Polymers 285: 119218
12. Yuste JR, Pozo JLD, Quetglás EG, Azanza JR (2006) Infecciones más comunes en el paciente trasplantado. Anales del Sistema Sanitario de Navarra 29
13. Zarb P, Coignard B, Griskeviciene J et al (2012). The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 17(46)
14. Zhou C, Yang Z, Xun X et al (2022) De novo strategy with engineering a multifunctional bacterial cellulose-based dressing for rapid healing of infected wounds. Bioactive Materials 13: 212–22
15. Zhou Y, Cai J, Wang X et al (2021) Distribution and resistance of pathogens in infected patients within 1 year after heart transplantation. International Journal of Infectious Diseases 103: 132-7
This article is excerpted the Wounds International 2024 | Volume: 15 Issue: 1 by Wound World.


