Electrical burns cause around 180,000 deaths a year and represent 5-8% of total burns in developed countries (World Health Organization, 2016). In adults, they occur during work activities; in children, they occur mainly at home. The injury severity depends on the intensity of the electrical current (determined by the source voltage and the resistance of the affected), compromised tissues in the patient’s body, and the duration of exposure. Although there are no specific parameters to therapeutic approach established in international literature, Gómez et al (2020) have shown that the use of multiple reconstructive techniques is mandatory due to the variety of affected areas, differing depth of burns and the need to prioritise coverage and early reconstruction to avoid growth and infection of exposed tissues, which would add complexity to the already severe trauma patients experience.
Wound management of electrical burns
Versus other types of burns, electrical burns lead to longer hospital stays, a greater number of surgical procedures and a higher probability of death. Wound management requires washing and debridement of the devitalised tissue, which should be done 72 hours after the burn occurred; evaluation of the progression of necrosis, which can cause infection; and finally the exclusion of sepsis (Castro et al, 2019).
Patient management, particularly in the case of large burns — defined by the American Burn Association as grade II burns covering greater than 25% of total body surface area (TBSA) — requires a multidisciplinary approach due to multiple systems being compromised secondary to the transit of the electrical current through the body tissues, generating necrosis. Burns create multiple local changes, such as injury to the inner coat of blood vessels, which generates the release of inflammatory mediators as a response, favouring vasoconstriction and thrombus formation.
This lack of oxygenation leads to progressive ischaemia at the level of the micro-circulation, generating necrosis, where the areas defined by Jackson (1947) can be identified: a central area with necrosis due to coagulation without cellular activity, surrounded by another area of lower intensity of damage with vascular stasis, and in which there is great metabolic activity of keratinocytes and Langerhans cells. As multiple references have shown (Castro et al 2019; Gómez et al, 2020), 60% of cases with vascular and nervous involvement have led to extremity amputation, in addition to inflammatory reactions generated at systemic level, characterised by the release of FNTA, cytokines and interleukins.
All these factors can generate complications when generating physiological healing, and we know that the epithelial approach is only part of the epithelialisation process. Other factors that may be involved, shown by Moffatt and Vowden (2008), are the extent of surrounding dermatitis, presence of scars, fibrosis, amount of exudate, oedema, concomitant diseases, malnutrition and mobility limitations, as well as the extent depth and loss of tissue that can affect the healing process, complicating the initial picture.
Epidermal growth factor
Epidermal growth factor (EGF) is a 53 amino acid polypeptide, first isolated by Dr Stanley Cohen from the submaxillary glands of mice in 1962. It stimulates the growth of fibroblasts, keratinocytes, and vascular endothelial cells, which contribute to forming healing tissue. As shown by López-Saura et al (2011), its action is triggered by interaction with specific receptors located on the cell membrane. Success has been reported in the regeneration of diabetic foot ulcers, an indication that has been widely studied and proven to have adequate efficacy. In other indications, potential benefits have also been proposed as adjuvant therapy on venous leg ulcers, secondary to varicose disease, by Dumantepe et al (2015) and Cacua and Giraldo (2019). Data on its use on burns are limited, but a study carried out on one of our patients with an electrical burn suggested that its administration could contribute to a valuable therapeutic strategy that improves the aesthetic and functional results of the patient with rapid epithelialisation and favourable evolution (Espitaleta et al, 2018). The EGF to be applied is that of the recombinant human (rhEGF) type, which shows efficacy, tolerability and safety: Esquirol-Caussa and Herrero-Vila (2016) have shown that bioidentical rhEGF can be made available in precise and stable concentrations and, therefore, is useful as a drug substance.
Case study
A 21-year-old male patient arrived at the emergency room after exposure to electrical current when handling high-voltage cables in his work as an industrial electrician. The initial check found multiple II and III degree burns across 20–30% total body surface area (TBSA), located in the submaxillary region of the neck [Figure 1], back of the chest at the level of the right scapular region, right hand and forearm.
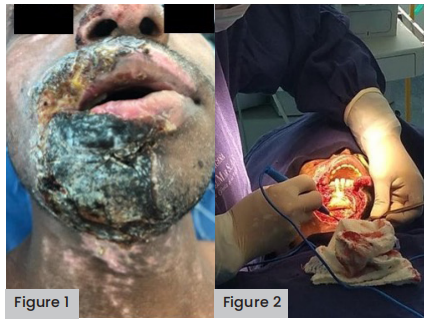
Figure 1 (far left). Multiple II and III-degree burns across 20–30% TBSA, located around the jaw and submaxillary region of the neck.
Figure 2 (left). Debridement plus
During the initial surgical management, an avulsive escharectomy and compound flaps were performed at distances, pedicled at different surgical stages and free flaps with a micro-vascular technique to cover tendons. Subsequently, treatment was started with five ampoules of nepidermin or rhEGF and Triticum vulgare gauze was applied as an adjuvant for wound hydration. The rhEGF vials were applied three times per week for 2 consecutive weeks to improve epithelialisation and decrease bone resorption in the jaw.
Long-term treatment
During recovery at home, 2 months after surgery, the patient developed soft tissue neck infections from common bacteria. Antibiotic therapy guided by antibiograms was prioritised, as well as placing flaps on the neck. Two months later, a mandibular fistula complication with fragmented bone extrusion occurred, and it was decided to apply five further vials of rhEGF to help close the fistula and stop bone resorption, which epithelised after 40 days.
The patient had hypertrophic and keloid scars, for which treatment was started with triamcinolone acetonide directly on the scar, every 2 weeks for four sessions, achieving a reduction of 70% of keloid scars. Topical creams with Allium cepa extract and antioxidants were continued twice daily for 3 months.
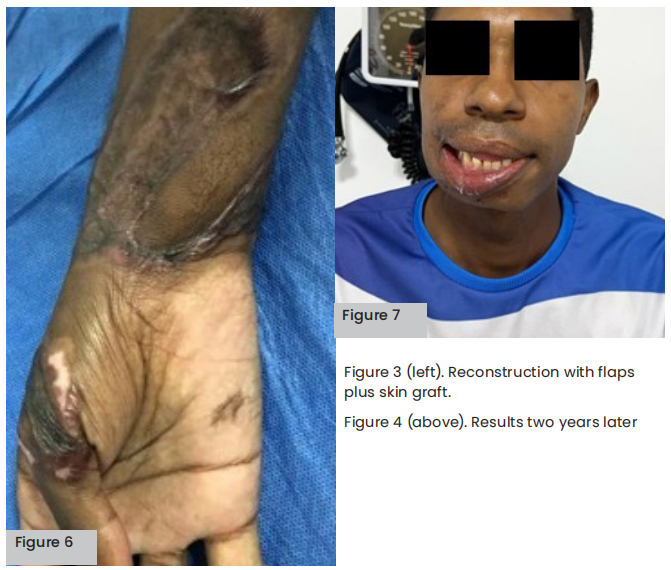
Three years later, hyaluronic acid was applied to the patient’s chin and tensile threads to improve his right lip corner and physical appearance. In addition, he has had follow-up panoramic X-rays to observe healing of the jaw: despite the patient’s teeth being weakened due to the injury, they are still intact 3 years later and, therefore, periodontal surgery follow-ups continue.
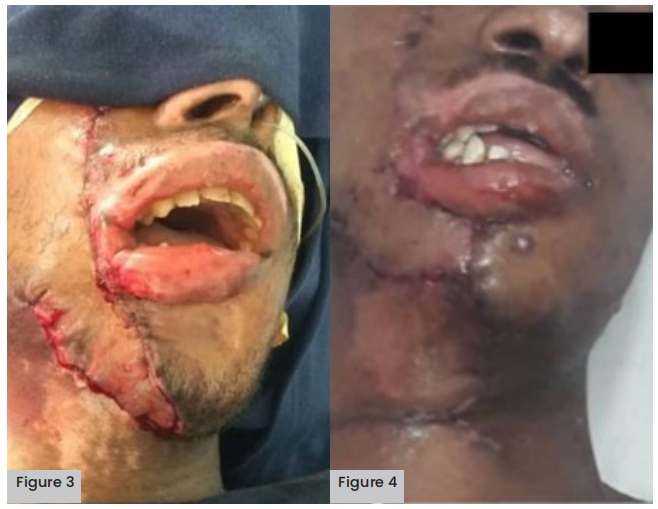
Figure 3 (above left). Flaps and skin grafts.
Figure 4 (above right). Neck advancement flap.
Initial approach
The approach during the first contact with the patient was that of ABCE emergency support as shown by González et al (2020): A — airway management with cervical spine control, B — breathing (ventilation), C — circulation and haemorrhage control, D — neurological deficits, E — exposure to and protection from the environment. Subsequent to ABCDE and after stabilisation of the patient, Chinome et al (2018) have shown that immediate surgical management should be considered if there is evidence of compartment syndrome or if it is imminent, or if there are alterations in ventilatory mechanics due to large areas of chest burn. Early escharectomy or tangential removal (from day three to five) is also part of the pillars of management, and finally coverage with skin grafting or flaps in the residual bloody areas as shown by multiple references (Koumbourlis, 2002; Arnoldo et al, 2006). Initial surgical management with fasciotomies and escharotomies decreases the requirement for extremity amputation, which in patients with this type of injury becomes as high as 40% in the upper extremities as shown by multiple references (Arnoldo et al, 2006; Bartley et al, 2019).
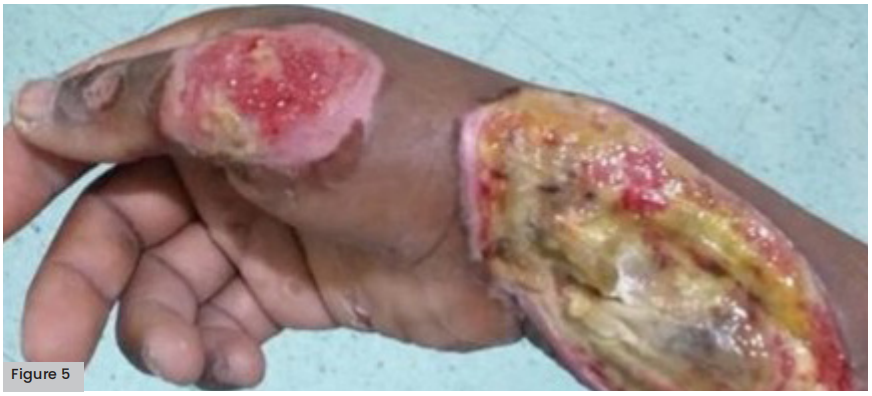
Figure 5 (opposite). Grade II burns on forearm and hand
Conclusion
In a review of human experience with 13 studies and a total of 1,924 participants, Esquirol-Caussa and Herrero-Vila (2017) concluded that the topical use of EGF and rhEGF contribute to reducing healing time and that the factors also have beneficial effects on pigmentation, flexibility, thickness and vascularization, without producing adverse effects. The review concludes that the use of growth factors may be an effective adjuvant to the treatment of burns. Several clinical trials show that EGF on second degree burns, as with chronic skin grafts and ulcers, speeds up skin regeneration and can prevent skin hyperpigmentation after laser treatment (Esquirol-Caussa and Herrero-Vila, 2017).
Fernández-Montequín et al (2009) specify that rhEGF plays an important role in regulating cell growth, proliferation and differentiation, which can be useful to improve wound healing — as we saw in the beneficial effect of the topical application of rhEGF in low-complexity neuropathic ulcers. However, as the topical application of rhEGF at the local level has already been seen in different aetiologies, it is not so beneficial in high-complexity ulcers due to the inflammatory environment that this type of ulcer presents. Fernández-Montequínet al (2009) have also shown that the intralesional and perilesional administration of this medicine can overcome this barrier by having the rhEGF reach its target directly, by stimulating fibroblast.
Studies have reported EGF and basic fibroblastic growth factor (bFGF) play a role in the mineralisation of the extracellular matrix, a complex process required for proper bone regeneration, which is one of the greatest challenges in regenerative dentistry. Angel Mosqueda (2015) has shown that a low concentration of EGF (10 ng/mL) is sufficient to induce morphological and phenotypic changes that are characteristic of osteoblastic cells. In addition, the balance between bone resorption and bone formation is influenced by a series of interrelated factors, such as genetic, mechanical, vascular, nutritional, hormonal and local. Within the latter, according to Hernández-Gil et al (2006), there are growth factors that act as modulators of cellular functions, fundamentally on cell growth, differentiation and proliferation. This could suggest, given the improvement in bone resorption in our patient, that the rhEGF used in this study aids in bone resorption. We recommend that new studies like this one be conducted with more patients to check our findings.
The available scientific evidence added to the rapid re-epithelialisation and the favourable evolution observed in our patient suggests that administering rhEGF in this type of injury could be a valuable therapeutic strategy to improve the final aesthetic and functional results of electrical burns. This confirms what was found by Espitaleta et al (2018) in a previous case report on this same pathology: that EGF improves aesthetic results in electrical burn patients in addition to functional aspects, with a rapid epithelialisation and a favourable progress.
References
1. Angel Mosqueda CD (2015) Aislamiento, Caracterización y Cultivo de Células Madre de Pulpa Dental Humana y Su Diferenciación a Linaje Osteogénico in vitro Utilizando Factor de Crecimiento Epidérmico y Factor de Crecimiento Fibroblástico Básico. Doctoral Thesis, Universidad Autónoma de Nuevo León. Available at: https://shorturl.at/HLWZ1 (accessed 23.02.2024)
2. Arnoldo B, Klein M, Gibran N (2006) Practice Guidelines for the Management of Electrical Injuries. J Burn Care Res 27(4): 439–47
3. Bartley CN, Atwell K, Purcell L et al (2019) Amputation Following Burn Injury. J Burn Care Res 40(4): 430–6
4. Cacua MT, Giraldo LF (2019) Experience with the use of perilesional and intralesional recombinant human epidermal growth factor (nepidermin) in the treatment of patients with chronic venous ulcers. Vasc Dis Manag 16(1): E3–E8
5. Castro L, Vargas S, Rueda J, García S (2019) Fisiopatología de las quemaduras eléctricas: artículo de revisión. Revista Chilena de Anestesia 48(2): 115–22
6. Chinome JEO, Jaimes YAP, Rosales SAV, Alviar JD (2018) Síndrome compartimental agudo en quemadura eléctrica. Revista de Ciencias Médicas 43(1): 35–8
7. Dumantepe M, Fazliogullari O, Seren M et al (2015) Efficacy of intralesional recombinant human epidermal growth factor in chronic diabetic foot ulcers. Growth Factors 33(2): 128–32
8. Espitaleta O, Montoya M, Barranco L (2018) Electrical burn: case report, multidisciplinary treatment and effectiveness of treatment with recombinant epidermal growth factor. Adv Plast Reconstr Surg 2(4): 230–33
9. Esquirol-Caussa J, Herrero-Vila E (2016) Un enfoque para el tratamiento de las úlceras de origen vascular: revisión y papel del factor de crecimiento epidérmico. Angiología 68(4): 322–30
10. Esquirol-Caussa J, Herrero-Vila E (2017) Epidermal Growth Factor (EGF) and silicone gels in wounds, burns and scars management: literature review. Cir Plást Iberolatinoam 43(4): 387–94
11. Fernández-Montequín JI, Betancourt BY, Leyva-Gonzalez
12. G et al (2009) Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: treatment up to complete wound closure. Int Wound J 6(1): 67–72
13. Gómez J, Rondón C, Tamayo W et al (2020) Manejo del gran quemado eléctrico y uso de colgajos musculares en hospital de IV nivel. Col Journal Cirugía Plástica y Reconstructiva 26(2): 39–44
14. González PD, Otero PP, Cabo JAV (2020) Manejo Avanzado del Paciente Politraumatizado. Editorial Medica Panamericana (Spain)
15. Hernández-Gil IFT, Gracia MAA, Pingarrón MdC, Jerez LB (2006) Physiological bases of bone regeneration II. The remodeling process. Med Oral Patol Oral Cir Bucal (Internet) 11(2): E151–7
16. Koumbourlis AC (2002) Electrical injuries. Crit Care Med 30(Suppl): S424–S430
17. López-Saura PA, Berlanga-Acosta J, Fernández-Montequín JI et al (2011) Intralesional human recombinant epidermal growth factor for the treatment of advanced diabetic foot ulcer: from proof of concept to confirmation of the efficacy and safety of the procedure, in: Dinh T (ed.) Global Perspective on Diabetic Foot Ulcerations. InTech, London: 217-38
18. Moffatt C, Vowden P et al (2008) Heridas de difícil cicatrización: un enforque integral. European Wound Management Association Positioning Document. Logroño: Spain. Available at: https://gneaupp.info/heridas-dedificil-cicatrizacion-un-enfoque-integral/ (accessed 23.02.2024)
19. World Health Organization (2016) Quemaduras. Geneva: WHO. Available at: http://www.who.int/es/news-room/ fact-sheets/detail/burns (accessed: 23.02.2024)
This article is excerpted from the Wounds International 2024 | Volume: 15 Issue: 1 by Wound World.


