Diabetes distress is an emotional response to living with diabetes and the burden of self-management, in which people experience feelings such as stress, guilt or denial (Kreider, 2017). It has been linked to worse health outcomes. Diabetes distress can fluctuate over time, and if left untreated, mild diabetes distress may develop into severe diabetes distress and/or depression. It is estimated that severe diabetes distress affects one in four people with type 1 diabetes and one in five of those with insulin-treated type 2 diabetes (Hendrieckx et al, 2019).
In 2014, the FreeStyle Libre (FSL) intermittently scanned continuous glucose monitoring system became available as a potential alternative to fingerprick self-monitoring of blood glucose. The sensor utilises wired enzyme technology to continuously measure interstitial glucose levels (see How to initiate and support continuous glucose monitoring). A reader or smartphone app can be used to scan the sensor and provides information on current and previous glucose levels and trends. The FSL has been updated by a new version, FreeStyle Libre 2 (FSL2) which, in addition to the above, has optional alarms to alert users when glucose levels fall outside their target range. More recently, following an update to the FSL app, the FSL2 sensor can send a glucose reading to the user’s phone every minute, effectively making it a realtime continuous glucose monitor.
In the UK, the use of FSL is not limited to people with type 1 diabetes; certain cohorts of people with type 2 diabetes also qualify for prescription of the FSL2 system. However, the eligibility criteria are open to interpretation. In Scotland, FSL2 is recommended in individuals with diabetes who are actively engaged in the management of their diabetes and who intensively manage their condition with multiple daily insulin injections or insulin pump therapy (Scottish Health Technologies Group, 2018).
Several studies have shown a statistically significant reduction in diabetes distress when using FSL compared with conventional fingerprick self-monitoring of blood glucose in people with type 2 diabetes who are on a basal–bolus insulin regimen (Haak et al, 2017; Ogawa et al, 2021). Furthermore, HbA1c has also been shown to significantly reduce within the first 6 months of using FSL in people with type 2 diabetes who were previously on multiple daily injections of insulin, with consequent improvements in treatment satisfaction and reductions in diabetes-related complications (Yaron et al, 2019; Eeg-Olofsson et al, 2023). Reducing diabetes distress can lead to an improvement in overall glycaemic control (Elotla et al, 2023).
Twice-daily pre-mixed insulin is an alternative insulin regimen used to treat type 2 diabetes for those who prefer a simpler regimen to basal–bolus insulin. The present service evaluation looks at the impact of using FSL2 on diabetes distress and HbA1c in people with type 2 diabetes who were on a twice-daily pre-mixed insulin regimen attending a pharmacist-led diabetes clinic in Scotland.
Methods
Ethical approval
As this was a service evaluation and not a research programme, ethical approval was not needed.
Data collection
People with type 2 diabetes on a twice-daily pre-mixed insulin regimen who had recently (within 3–6 months) started using FSL2 were included in this audit. The participants were identified from two GP practices by running a search on the EMIS electronic patient record system. Altogether, ten participants were identified.
Diabetes distress was calculated using the Problem Areas In Diabetes (PAID) scale, a widely used and validated tool to measure diabetes distress (de Wit et al, 2022). The tool comprises 20 questions pertaining to problem areas, such as not having clear goals for the management of diabetes and worrying about the complications of diabetes.
Before sending out the PAID questionnaire to participants, consent was obtained via a telephone call. Following consent, the PAID questionnaires were posted along with clear instructions on how to complete; the participants completed the same questionnaire twice: once to detail how they felt about their diabetes before they started using FSL2, and the second to detail how they felt about their diabetes since they started using FSL2. A stamped addressed envelope was also included to allow ease in returning the completed questionnaire.
Participants indicated which of the 20 PAID items was currently a problem for them, with scores ranging from 0 (not a problem) to 4 (a serious problem). The score of each item was summed and then multiplied by 1.25, to generate a total score out of 100. Any total score of 40 or above was deemed to be severe diabetes distress, while any score of 3 or 4 on individual items were deemed to be moderate to severe diabetes distress.
Results
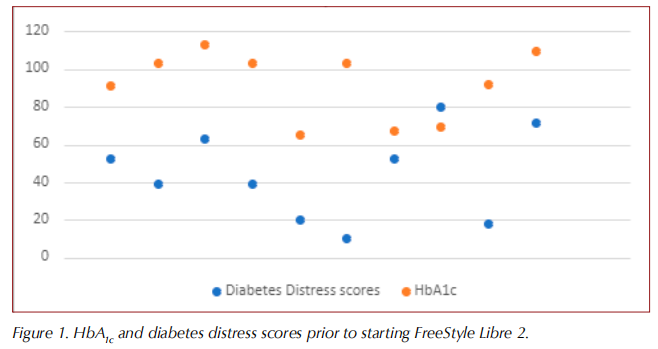
Table 1 shows the clinical characteristics of the study population, which consisted of ten participants with type 2 diabetes. The median age of the study population was 73.2 years and 80% were male. The median duration of diabetes was 19.3 years.
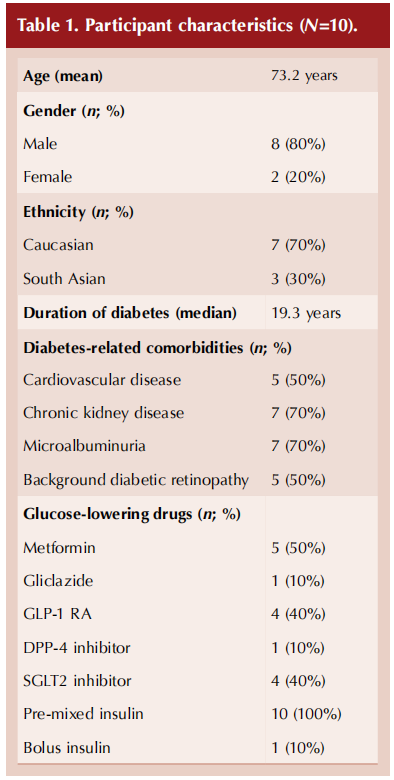
Table 2 (overleaf) compares PAID scores and HbA1c before and after commencing the FSL2. The mean PAID score decreased from 44.4 to 24.0 (a 46.4% reduction) after initiating FSL2. The prevalence of severe diabetes distress reduced from 50% to 20%, while the rate of moderate to severe diabetes distress reduced by a relative 55%. Average HbA1c also reduced by 18 mmol/mol (1.7%).
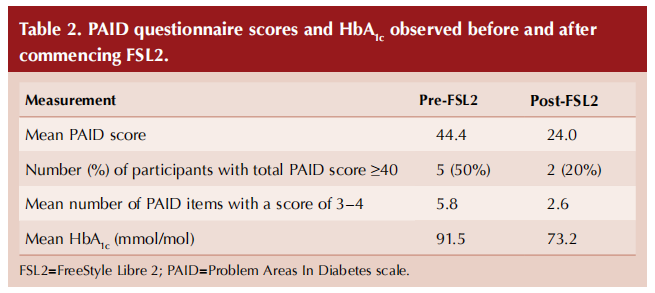
Visually, no relationship between HbA1c and level of diabetes distress was apparent (Figure 1).
Discussion
This service evaluation looked at the impact of using FSL2 on diabetes distress and HbA1c in people with type 2 diabetes who were on a twice-daily pre-mixed insulin regimen and attending a pharmacist-led diabetes clinic.
The results show that, after 3–6 months of using FSL2, reductions were seen in both diabetes distress and HbA1c. The mean diabetes distress score reduced by 46.4%, and a reduction in moderate to severe diabetes distress was seen. Average HbA1c also fell.
There was no apparent relationship between HbA1c and levels of diabetes distress, suggesting that optimal HbA1c is not necessarily an indicator of low diabetes distress. This finding is comparable with other published studies (Gonzalez et al, 2016; Fayed et al, 2022).
To the author’s knowledge, this is the first research to explore the effect of using FSL2 on diabetes distress and HbA1c in people with type 2 diabetes who are on twice-daily pre-mixed insulin. However, due to the small cohort of ten participants, the study lacked power for statistical analysis. Furthermore, as PAID scores before using the FSL2 were based on the participants’ recall, this may have introduced biases such as recall and confirmation bias. Finally, as the dataset was small and limited to two GP practices, the results may not be generalisable to the wider UK population.
Although other studies have not compared the effect of diabetes distress and HbA1c in people with type 2 diabetes who are on twice-daily pre-mixed insulin who are using FSL2, the findings of this service evaluation are comparable with studies conducted in people with type 2 diabetes using multiple daily injections of insulin.
The results suggest that people with type 2 diabetes on twice-daily pre-mixed insulin regimens will benefit greatly from using FSL2, as a result of improved quality of life and, given the observed reductions in HbA1c, diabetes outcomes.
Further research needs to be performed in this group of people, as currently the criteria for use of FSL2 are limited to those with type 1 diabetes and those with type 2 diabetes who are on multiple daily insulin injections. Local and national guidelines may need to be amended to include the use of FSL2 in people with type 2 diabetes on twice-daily pre-mixed insulin.
References
1. de Wit M, Pouwer F, Snoek FJ (2022) How to identify clinically significant diabetes distress using the Problem Areas In Diabetes (PAID) scale in adults with diabetes treated in primary or secondary care? Evidence for new cut points based on latent class analyses. BMJ Open 12: e056304
2. Eeg-Olofsson K, Svensson AM, Franzén S et al (2023) Real-world study of flash glucose monitoring among adults with type 2 diabetes within the Swedish National Diabetes Register. Diab Vasc Dis Res 20: 14791641211067418
3. Elotla SF, Fouad AM, Mohamed SF et al (2023) Association between diabetes-related distress and glycemic control in primary care patients with type 2 diabetes during the coronavirus disease 2019 (COVID-19) pandemic in Egypt. J Family Community Med 30: 42–50
4. Fayed A, AlRadini F, Alzuhairi RM et al (2022) Relation between diabetes related distress and glycemic control: The mediating effect of adherence to treatment. Prim Care Diabetes 16: 293–300
5. Gonzalez JS, Kane NS, Binko DH et al (2016) Tangled Up in Blue: Unraveling the links between emotional distress and treatment adherence in type 2 diabetes. Diabetes Care 39: 2182–9
6. Haak T, Hanaire H, Ajjan R et al (2017) Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: A multicenter, open-label randomized controlled trial. Diabetes Ther 8: 55–73
7. Hendrieckx C, Halliday JA, Beeney LJ, Speight J (2019) Diabetes and emotional health: A practical guide for healthcare professionals supporting adults with type 1 and type 2 diabetes. Diabetes UK, London. Available at: https://bit.ly/3PgiLyA
8. Kreider KE (2017) Diabetes distress or major depressive disorder? A practical approach to diagnosing and treating psychological comorbidities of diabetes. Diabetes Ther 8: 1–7
References (continued)
1. Ogawa W, Hirota Y, Osonoi T et al (2021) Effect of the FreeStyle Libre™ flash glucose monitoring system on glycemic control in individuals with type 2 diabetes treated with basal– bolus insulin therapy: An open label, prospective, multicenter trial in Japan. J Diabetes Investig 12: 82–90
2. Scottish Health Technologies Group (2018) Advice statement: Freestyle Libre® flash glucose monitoring. Available at: https:// shtg.scot/our-advice/freestyle libre-flash-glucose-monitoring/
3. Yaron M, Roitman E, Aharon-Hananel G et al (2019) Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 42: 1178–84
This article is excerpted from the Diabetes & Primary Care Vol 26 No 2 2024 by Wound World.


