Non-alcoholic fatty liver disease (NAFLD) comprises a range of liver pathologies including liver steatosis with or without inflammation, which can progress to fibrosis, cirrhosis and increased risk of hepatocellular cancer. Co-existing type 2 diabetes increases the risk of liver disease progression and worse outcomes. People with NAFLD are at increased risk of cardiovascular disease, liver disease progression and all-cause death (Kim et al, 2024), with cardiovascular disease (CVD) being the commonest cause of death.
More recently, the term NAFLD has been changed by some organisations to “metabolic dysfunction-associated steatotic liver disease” (MASLD) for those with steatotic liver disease who have at least one cardiometabolic risk factor associated with insulin resistance (prediabetes, diabetes, hyperlipidaemia or hypertension) and who are without other identified causes of liver disease such as high alcohol intake (defined as current or recent intake of >21 standard drinks per week in men and >14 standards drinks in women). The term MetALD – metabolic dysfunction and alcoholic liver disease – is used for those with alcohol intake higher than in MASLD but less than that likely to cause alcoholic liver disease.
The Fibrosis-4 Index (Fib-4) has been shown to perform similarly or better than other liver fibrosis biomarkers, including the enhanced liver fibrosis (ELF) test, and is therefore recommended in many guidelines as the first-line non-invasive tool for assessing advanced liver fibrosis risk in people with or at risk of developing NAFLD. Recent guidance from the ADA in the 2024 Standards of Care suggests that, in people with obesity and diabetes, Fib-4 should be used to screen for advanced liver fibrosis in high-risk groups even if liver function tests are normal, as otherwise many people with fibrosis would remain undiagnosed (ADA Professional Practice Committee, 2024).
Fib-4 is calculated using the formula (where AST=aspartate aminotransferase and ALT=alanine transaminase):
(Age × AST) ÷ (Platelet count × √ALT)
Calculators are readily available and are embedded in some electronic health records, making Fib-4 simple to calculate and code. AST and ALT should have been measured on the same day and platelets within 30 days previously.
A Fib-4 score of <1.30 indicates low risk of advanced fibrosis, 1.3–2.67 indicates indeterminate risk, and >2.67 indicates high risk. In low-risk cases, a repeat Fib-4 score is recommended after 1–3 years depending on ongoing risk (Rinella et al, 2023). Those with an indeterminate or high Fib-4 should receive liver stiffness measurement with transient elastography scanning or, if this is not available, an ELF blood test. Referral into secondary care may be required to access scanning or ELF tests, and is required if either of these tests show significant fibrosis risk.
The present study
This study, published in The Lancet Regional Health – Europe, included participants in the UK CPRD GOLD database who were aged ≥18 years with obesity and/or type 2 diabetes who had at least one Fib-4 measurement, excluding those with types of liver disease other than MASLD, those with alcohol-related problems and those who were on drugs that might induce chronic liver disease. In addition, those with known liver or cardiovascular events prior to baseline were excluded. Study participants were followed for a mean of 10 person-years or until their first event.
The three composite endpoints were defined as:
●Liver events: Liver-related hospitalisation or death from hepatocellular carcinoma, liver transplant, liver failure, liver cirrhosis, portal hypertension or hepatic decompensation (ascites, hepatorenal syndrome, hepatic encephalopathy, varices with or without bleeding).
●Cardiovascular events: CVD-related hospitalisation or death from myocardial infarction, stroke, unstable angina or heart failure, and coronary revascularisation.
●All-cause mortality.
Results
Due to exclusions for baseline events, a total of 44 311, 40 565 and 44 477 people were included in the analyses for liver, CVD and mortality events, respectively. In total, there were 979 liver events, 6002 CVD events and 8971 deaths. Cumulative incidences of the liver, CVD and mortality endpoints in the low-, indeterminate- and high-risk Fib-4 groups are shown in Table 1.
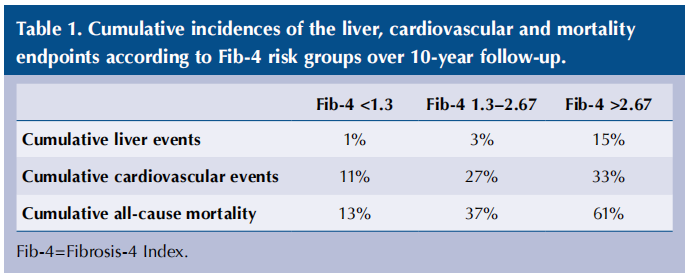
Compared with low-risk Fib-4 scores at baseline, the hazard ratios for the indeterminate risk group were 2.82 for liver events, 2.97 for cardiovascular events and 3.44 for all-cause mortality; however, when adjusted for age, sex and Framingham cardiovascular risk score at baseline, these hazard ratios were not significantly different. For those in the high-risk Fib-4 group, the hazard ratios compared with the low-risk group were 18.4 for liver events, 4.73 for cardiovascular events and 7.25 for all-cause mortality, and these remained significant even when adjusted for age, sex and Framingham risk score.
More than 20 000 participants had a suitable repeat Fib-4 result at 12–15 months. In the liver event follow-up group, 10.5% increased their Fib-4 score over the 12 months, while 8.2% decreased it. As might be expected, the risk of liver events increased amongst those whose Fib-4 increased, and decreased amongst those in whom Fib-4 scores fell, irrespective of baseline Fib-4 risk category. Similar findings were found for cardiovascular risk and all-cause mortality.
Implications for practice
There have been many recent clinical papers reminding us of the importance of primary care assessment, lifestyle advice and referral for further investigation of people with potential NAFLD/MASLD, particularly amongst those with co-existing type 2 diabetes, which greatly increases the liver, cardiovascular and mortality risks. Lifestyle changes aiming to reduce weight and waist circumference remain the mainstay of treatment (Zelber-Sagi and Moore, 2024). Although GLP-1 receptor agonists such as semaglutide and the dual incretin agonist tirzepatide produce significant weight loss and are being studied as treatments for NAFLD, none are yet licensed for this indication in the UK. Pioglitazone has data supporting a beneficial impact but is also not licensed for this indication, and care is required due to adverse effects including weight gain and fracture risk, and it cannot be used in those with heart failure.
So what can we do? As suggested, we can use Fib-4 measurements annually to monitor those with low initial scores and help motivate them to make lifestyle changes, and to identify those who require referral for further assessment or management. Since people with NAFLD are at high cardiovascular risk, we can help them reduce this risk with lifestyle changes, statins and optimal blood pressure and glucose control. Statins are not contraindicated in most people with NAFLD (ADA Professional Practice Committee, 2024), but it is important to follow the national guidance (see Khatib and Neely, 2023) and seek advice from secondary care if there is doubt (e.g. ALT >3-times the upper limit of normal) and consider other safe treatments.
A useful summary, “Practical lifestyle management of NAFLD for busy clinicians” published in Diabetes Spectrum, covers everything we need to know (Zelber-Sagi and Moore, 2024).
The paper provides a one-page lifestyle checklist which I now use in consultations, and which I have adapted as a take-away summary for people with NAFLD. Weight loss recommendations include the following:
● ≥5% for people with NAFLD and overweight or obesity, to reduce risk of progression.
● 7–10% for reduction of non-alcoholic steatohepatitis and fibrosis.
● 3–5% in those with normal weight to reduce abdominal obesity.
Referral to Tier 2, 3 or 4 weight management services according to local pathways will provide the support needed to achieve such weight reductions.
NAFLD results in significantly increased risk of CVD, liver disease progression and mortality, and interventions can reduce these risks; however, current rates of diagnosis and referral for further management remain low in the UK. By ensuring that we request AST, ALT and platelets annually when organising routine diabetes blood tests, and by taking the time to calculate the Fib-4 score until it is calculated routinely by our electronic record software, we can help ensure better diagnosis and management and increase quantity and quality of life.
References
1. American Diabetes Association Professional Practice Committee (2024) 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Care in Diabetes – 2024. Diabetes Care 47(Suppl 1): S52–76
2. Khatib R, Neely D; Accelerated Access Collaborative (2023) Summary of national guidance for lipid management for primary and secondary prevention of cardiovascular disease. NHS England. Available at: https://bit.ly/4aSmT1b
3. Kim KS, Hong S, Han K, Park CY (2024) Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: Nationwide population based study. BMJ 384: e076388
4. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS et al (2023) AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77: 1797–835
5. Zelber-Sagi S, Moore JB (2024) Practical lifestyle management of nonalcoholic fatty liver disease for busy clinicians. Diabetes Spectr 37: 39–47
This article is from the Diabetes & Primary Care Vol 26 No 2 2024 by Wound World.


