The incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are released by the K and L cells of the gut wall in response to food intake, and they have effects at distant sites such as the pancreas, brain and in adipose tissue. Incretin hormone levels are reduced in people with type 2 diabetes. Tirzepatide, the first single-molecule, dual GIP and GLP-1 receptor agonist, is licensed in the UK for the treatment of both type 2 diabetes and obesity, and has been demonstrated to slow gastric emptying, increase insulin sensitivity and glucose-dependent insulin secretion, and decrease glucagon secretion and appetite.
The NICE TA924 Technology Appraisal, published in October 2023, makes recommendations for tirzepatide therapy in those with type 2 diabetes, positioning tirzepatide similarly to the GLP-1 receptor agonists, alongside diet and exercise, when triple therapy with metformin and two other oral drugs is ineffective, not tolerated or contraindicated, and either:
● BMI is 35 kg/m2 or more, or
l● BMI is <35 kg/m2 and insulin therapy would have significant occupational implications, or weight loss would benefit other significant obesity-related complications.
BMI thresholds are reduced (usually by 2.5 kg/m2 ) for people from South Asian, Chinese, other Asian, Middle Eastern, Black African or African–Caribbean family backgrounds (NICE, 2023).
This is a more restricted positioning than tirzepatide’s licence, which allows its use for the treatment of adults with insufficiently controlled type 2 diabetes as monotherapy in addition to diet and exercise when metformin is considered inappropriate due to intolerance or contraindications, or as an addition to other glucose-lowering treatment.
For weight management, including weight loss and weight maintenance, tirzepatide is licensed as an adjunct to a reduced-calorie diet and increased physical activity in adults with an initial BMI of ≥30 kg/m2 (obesity) or ≥27 to <30 kg/m2 (overweight) in the presence of at least one weight-related comorbid condition (e.g. hypertension, dyslipidaemia, obstructive sleep apnoea, cardiovascular disease, prediabetes or type 2 diabetes). NICE guidance on the use of tirzepatide for obesity/weight management is expected in March 2024.
Tirzepatide is administered by once-weekly injection, starting with 2.5 mg weekly and increasing after 4 weeks to 5 mg weekly. Further increases in 2.5-mg increments should take place at 4-weekly intervals if higher doses are needed; thus, titration to the full 15 mg dose will take at least 20 weeks.
Clinical effects of tirzepatide
In their recent review of tirzepatide in type 2 diabetes, MacIsaac and colleagues challenge currently recommended guidelines, particularly glycaemic treatment targets, now that normoglycaemia can be achieved safely in significant numbers of people. Since the benefits of tight glycaemic control early in the disease are well documented, and sustained weight loss is recognised as an important goal in those with obesity and type 2 diabetes, the authors argue that guidelines may need to change to harness the potential benefits of tirzepatide, other incretin co-agonists and other combination drugs in development.
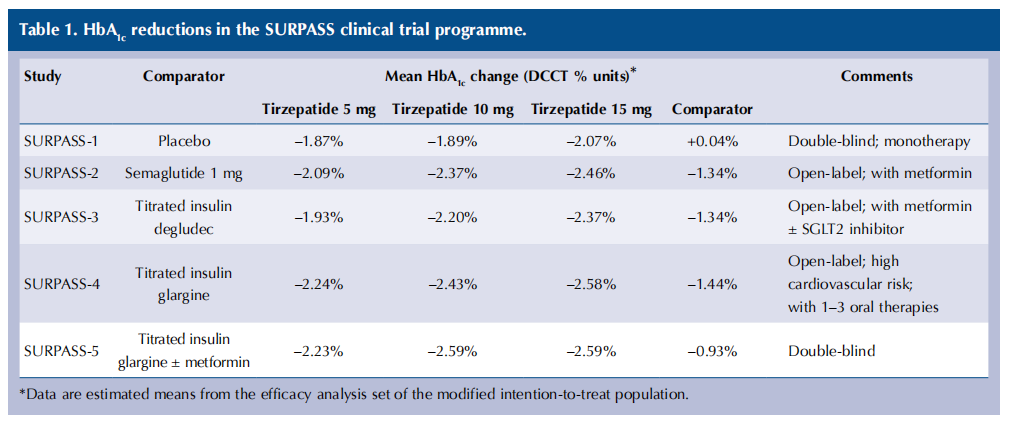
The SURPASS phase 3 clinical trial programme explored the efficacy and safety of tirzepatide in people with type 2 diabetes, and demonstrated dose-dependent reductions in HbA1c of 20–28 mmol/mol in those treated with tirzepatide: greater reductions than those achieved with semaglutide (Frías et al, 2021), insulin degludec (Ludvik et al, 2021) and insulin glargine (Del Prato et al, 2021), with reductions sustained up to 2 years in SURPASS-4.
Reductions in weight and waist circumference achieved in the SURPASS studies were significantly greater in those treated with tirzepatide versus the comparator drugs, with weight loss of ≥15% achieved by 27–43% of trial participants.
Tirzepatide was also associated with dose-dependent positive changes in lipids, with a reduction in triglycerides and increases in HDL cholesterol. In the high-cardiovascular-risk population in SURPASS-4, improvements in total, LDL and HDL cholesterol and triglycerides were seen in those treated with tirzepatide, greater than in those treated with insulin glargine.
HbA1c reductions in the SURPASS studies in type 2 diabetes are summarised in Table 1. There is a small dose-dependent increase in HbA1c reduction with the higher doses. Incremental weight reduction occurred with the higher doses in the SURMOUNT programme of weight management studies.
As would be expected, gastrointestinal events, usually mild to moderate in severity and occurring mostly during dose titration, were the most common adverse events, and occurred with similar frequency with tirzepatide (40–45% in SURPASS-2) to that seen with semaglutide (41.2%). Adverse events leading to study discontinuation occurred in 3–11% of those treated with tirzepatide, 3% with placebo, 4% with semaglutide and 1–5% with basal insulins.
Hypoglycaemia (blood glucose <3 mmol/L) occurred infrequently across the SURPASS programme and, as expected, the risk was greater in those also prescribed sulfonylureas or insulin. Hence, dose reductions in sulfonylureas and insulin are important considerations when initiating or titrating tirzepatide.
SURPASS-4 confirmed no significant increase in risk of cardiovascular disease with tirzepatide in a high-risk population, and a pre-specified meta-analysis demonstrated a hazard ratio of 0.8 for major adverse cardiovascular events (cardiovascular death, non-fatal stroke or myocardial infarction, or hospitalisation for unstable angina) over 55 weeks. Longer follow-up in the ongoing SURPASS-CVOT trial, comparing tirzepatide with dulaglutide, is needed to confirm if there is a cardiovascular benefit with tirzepatide, as seen with liraglutide, dulaglutide and semaglutide. No cases of medullary thyroid cancer occurred in the SURPASS programme, and pancreatitis, gallstone disease and diabetic retinopathy occurred in <1% of participants.
Patient-reported outcomes
Carefully selected patient-reported outcome (PRO) instruments (standardised questionnaires designed to understand the patient experience) were administered at baseline and at 40 or 52 weeks across the SURPASS clinical trial programme (Boye et al, 2023). Since tirzepatide is the first in a new drug class, it was deemed particularly important to understand the experience of users. Tirzepatide improved participants’ quality of life, as measured by general health and weightrelated PROs, and resulted in significantly greater improvements than comparators for most measured PROs in each of the five studies. Tirzepatide 15 mg was associated with significantly greater improvements in physical functioning scores compared to comparators, and generally resulted in greater improvements in quality of life.
Boye and colleagues remind us that people are more likely to adhere to therapies that they value, likely resulting in improved clinical outcomes, and that people place value on various attributes of treatment, including efficacy, safety and ease of use. Interestingly, despite clinician beliefs, other studies have demonstrated that oral therapy is not always preferred over injectable therapy.
These PROs were administered in a clinical trial setting, and the authors call for real-world PRO research to understand patient experience in tirzepatide prescribing settings.
Conclusions
With the imminent availability of tirzepatide in the UK for treatment of people with type 2 diabetes, this review provides a timely and thought-provoking discussion of the possible future and wider impact of tirzepatide on diabetes complications, particularly once dedicated cardiovascular and renal studies are available. With the same tirzepatide dosing licensed for glycaemic control and weight reduction, this may help minimise the challenges we face with limited Tier 3 weight management service access, finally allowing people with type 2 diabetes and obesity access to long-term, effective weight management drug therapy as well as highly effective glycaemic management.
Those of us adhering strictly to recommendations not to initiate GLP-1 receptor agonists during the shortage over the last 6 months, to avoid jeopardising supplies to those already being treated, will already have identified people with type 2 diabetes who would benefit from the glucose-lowering and weight-reducing effects of tirzepatide. I am very much looking forward to being able to offer tirzepatide therapy in my clinics as soon as supplies are consistently available in the UK.
References
1. Boye KS, Thieu VT, Sapin H et al (2023) Patient-reported outcomes in people with type 2 diabetes receiving tirzepatide in the SURPASS clinical trial programme. Diabetes Ther 14: 1833–52
2. Del Prato S, Kahn SE, Pavo I et al; SURPASS-4 investigators (2021). Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 398: 1811–24
3. Frías JP, Davies MJ, Rosenstock J et al; SURPASS-2 investigators (2021) Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 385: 503–15
4. Ludvik B, Giorgino F, Jódar E et al (2021) Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 398: 583–98
This article is excerpted from the Diabetes & Primary Care Vol 25 No 6 2023 by Wound World.


