INTRODUCTION
Skin is a multilayer organ that covers the surface of the human body and has the functions of defending the body against bacteria attacks, preventing body dehydration, and feeling external temperature [1,2]. However, skin is susceptible to injury from multiple factors such as cullet and knives [3]. Once the skin suffers an injury, it often needs wound dressing to achieve rapid closure and accelerate wound healing [4]. Due to the low cost and good hemostatic properties, gauze is the most commonly used wound dressing [5,6]. Unfortunately, gauze often causes severe secondary damage to the injured wound during gauze changes because the newly grown tissue is adhered tightly by gauze [4,7,8]. In addition, studies have shown that a moist healing environment facilitates the migration and proliferation of endothelial cells, fibroblasts, and epithelial cells, whereas traditional gauzes provide a dry healing environment, which is not favorable for wound healing [9–11].
Due to the advantages of high water content, biophysical similarity to natural tissue, and good tissue adhesion, adhesive hydrogels as wound dressings have aroused the interest of many researchers [12,13]. Adhesive hydrogels can not only adhere to the wound to close it and stop bleeding, but also act as a physical barrier to isolate the external environment and provide a moist environment to accelerate wound healing [14,15]. Tannic acid (TA), a natural polyphenol extracted from various plants, has valuable properties such as antibacterial and anti-inflammation abilities [16–18]. Importantly, TA can react with many polymers by hydrogen bonding because of its availability of catechol groups [19,20]. Since TA-based hydrogels with good adhesion ability can be obtained simply by mixing TA and polymers, such hydrogels are widely used in wound healing [18,20,21]. Nevertheless, almost all TA-based adhesive hydrogels typically dehydrate and shrink when exposed to open air, leading to the loss of flexibility and the moist environment, as well as causing unnecessary secondary damage to the newly formed tissue during dressing changes, which extremely limits their clinical applications [22,23].
Much research shows that the addition of highly hydrated salts or excess organic solvents can effectively improve the water retention properties of hydrogels, but these methods usually result in high osmotic pressure and poor biocompatibility [24–26]. Currently, a new strategy to improve the water retention capacity of hydrogel is to encapsulate the hydrogel with a hydrophobic coating [27–29]. Especially, the hydrophobic polyurethane diacrylate (PUA) coating polymerized from urethane diacrylate (UDA) using an “inside-out” technology can encapsulate a wide variety of hydrogels with various types and shapes easily, thus, preventing the evaporation of water from the hydrogel [27]. But this strategy has not yet been used in hydrogel wound dressings. We hypothesize that good water-retaining ability can also be obtained with adhesive hydrogels by encapsulating surfaces that do not contact to the wound with the PUA coating.
Recently, the method of on-demand removal has often been used to decrease secondary damage [4,30,31]. For example, Jiang et al. [30] developed a skin-friendly and painless removable adhesive hydrogel patch composed of polymerized gallic acid and gelatin methacryloyl (GelMA). The patch could adhere tightly to the warm skin and be removed on-demand through the shrinkage of the thermoresponsive GelMA chain after placing an ice bag above the patch. Based on the pH-sensitive coordination bond (catechol-Fe) and dynamic Schiff base bonds, Liang et al. [31] developed an adhesive hydrogel with ondemand removal or dissolution after the intervention of deferoxamine mesylate (DFO) solution. Generally, the on-demand removal of adhesive hydrogels was triggered by additional factors, which is inconvenient in practical application. In this study, we designed an adhesive hydrogel, whose adhesion energy was higher than its fracture energy, allowing it to be separated into parts upon peeling, and causing little wound damage during dressing changes, which has barely been reported.
In this work, a PUA-coated methacrylated silk fibroin/TA (SFMA/TA (ST)) hydrogel wound dressing with good tissue adhesion, long-lasting water retention, and separable abilities was prepared (Fig. 1). Firstly, SF was modified with glycidyl methacrylate (GMA) to obtain the SFMA, followed by preparing the SFMA hydrogel by ultraviolet (UV) crosslinking [32]. Then, the SFMA hydrogel was immersed in TA solution, and by varying the TA concentration and immersion time, the adhesion energy of the ST hydrogel was adjusted and optimized to ensure that the hydrogel had good tissue adhesion and separable abilities. Next, a PUA coating encapsulated the ST hydrogel to prepare a PUA-coated ST hydrogel with improved water retention capability. In addition, the swelling ratio, blood coagulation, in vivo hemostasis, and biocompatibility of the hydrogel were evaluated. Finally, by establishing a full-thickness model of rats, the effect of the PUA-coated ST hydrogel on promoting wound healing efficiency was examined.
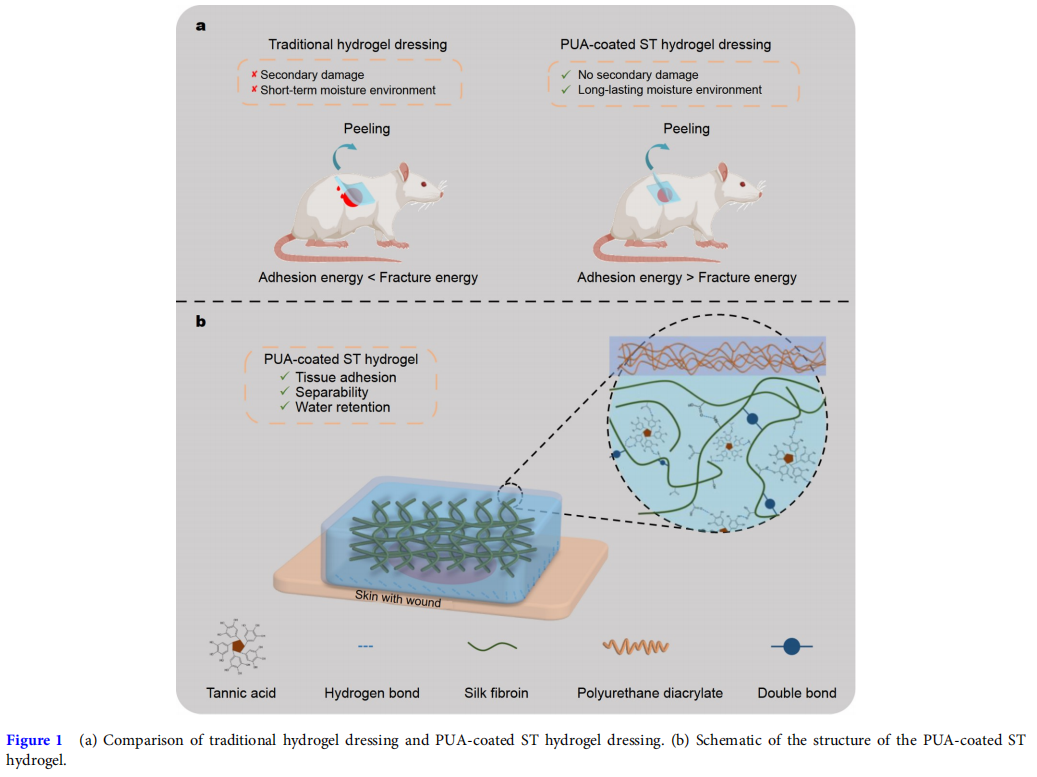
RESULTS AND DISCUSSION
Adhesion properties of the hydrogel
Silk is a natural protein polymer that has been approved for medical use by the U.S. Food and Drug Administration (FDA) [33]. SF was processed from mulberry silk, which possesses excellent biocompatibility, controllable biodegradability, remarkable mechanical strength, and low immunogenicity [32,34,35]. Most importantly, the SF had remarkable mechanical strength and could induce the formation of thrombin to accelerate blood coagulation [17,35,36]. Therefore, SF has been widely used for wound healing [14,17,37]. After being modified by GMA, the UV-cross-linkable SFMA was synthesized (Fig. S1a). As shown in Fig. S1b, new peaks (in the region of 5.8–6.2 ppm) ascribed to C=C double bonds could be observed in the 1 H nuclear magnetic resonance (NMR) spectrum of SFMA, indicating the successful modification. With the presence of lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as a photoinitiator, SFMA hydrogel was formed by exposing 15 wt% SFMA solution to UV light for 15 s. However, the SFMA hydrogel had almost no adhesion properties. TA, an FDAapproved polyphenol from nature involving abundant pyrogallol and catechol groups, allows strong adhesion between hydrogels and various materials through hydrogen bonding, π–π interaction, and metal chelation (Fig. 2a) [14,34,38]. By immersing SFMA hydrogel in TA solution, the ST hydrogel with adhesive properties could be obtained [17].
The 180-degree peel test was applied to quantify the adhesion energy of the SFMA/TA hydrogel. As shown in Fig. 2c, d, with the increasing concentration of TA and immersion time, the adhesion energy increased initially and then decreased. When the concentration of TA was 5 wt% and the immersion time was 2 h, the adhesion energy of the ST hydrogel reached a peak (52.6 ± 3.8 J m−2 ). The ST hydrogel with peak adhesion energy was selected to do next experiments. Moreover, this ST hydrogel could tightly adhere to the fingers with different bending angles (0°, 45°, 90°, and 135°) and to other materials such as rubber, metal, glass, and plastic (Fig. 2b). Interestingly, this hydrogel adhered to fingers could be separated upon peeling and then leave a residual adhesive to the skin (Fig. 2e), possibly because the adhesion energy of this hydrogel was higher than its fracture energy [39]. The next fracture energy test showed that the fracture energy of the hydrogel was 32.7 ± 2.4 J m−2 , which was lower than its adhesion energy. Meanwhile, the inseparable control group was obtained by adding 10 wt% methacrylated polyethylene glycol (PEGMA) to the ST hydrogel to increase its mechanical properties [40]. Fig. S1c shows the 1 H NMR spectrum of PEGMA, and the degree of substitution of methacrylate groups in PEGMA was approximately 65.83%. Similar to ST hydrogel, after being immersed in 5 wt% TA solution for 2 h, the PEGMA/ST (PST) hydrogel could reach a peak of adhesion energy (55.9 ± 6.6 J m−2 ), which was lower than its fracture energy (228.7 ± 44.5 J m−2 ) (Fig. S2). Contrary to the ST hydrogel, the PST hydrogel was inseparable upon peeling (Fig. 2f). Based on the above data, this separable ST hydrogel might be suitable as wound dressing to reduce the damage to the wound during dressing changes.
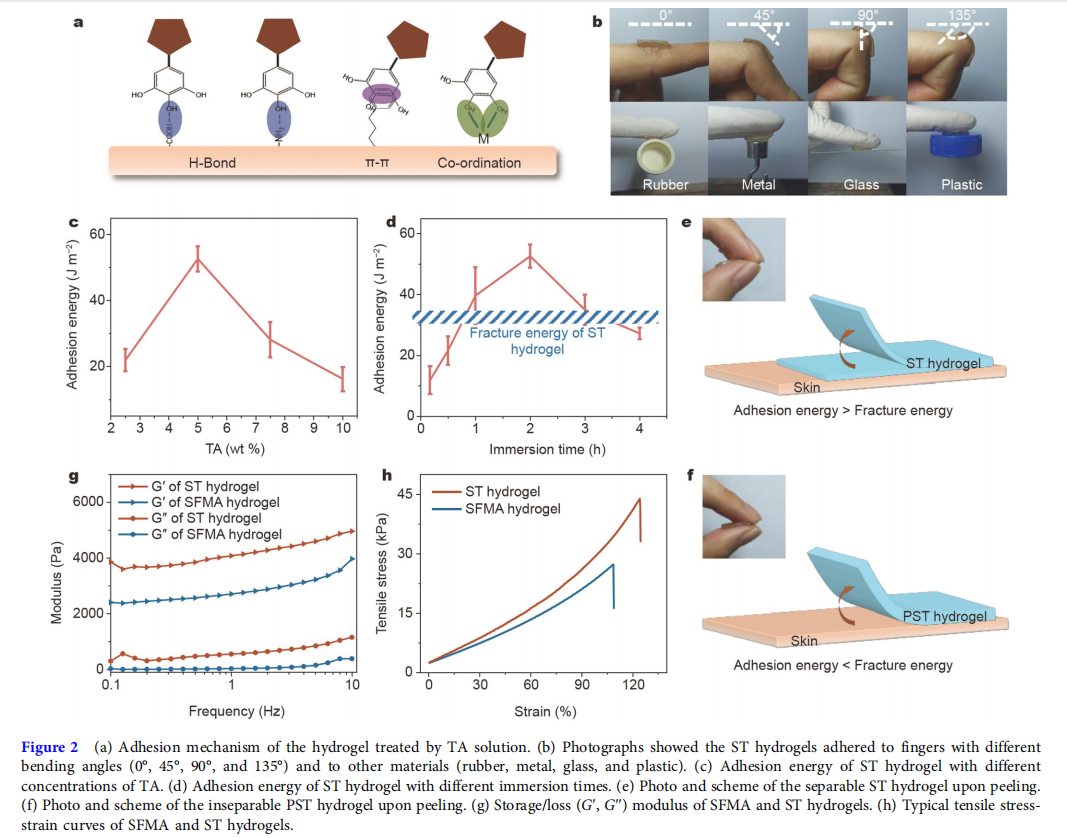
Mechanical properties of the hydrogel
Not only increasing the adhesion energy, but also the addition of TA could enhance the mechanical strength of the ST hydrogel due to the hydrogen bonding interactions between SFMA chains and TA [14,17]. This interaction endowed the ST hydrogel with a smaller pore size than that of the SFMA hydrogel (Fig. S3). To determine the rheological properties of SFMA and ST hydrogels, a 1% strain frequency (0.1–10 Hz) sweep test was carried out to monitor the G′ and G′′ after the hydrogels were formed. As shown in Fig. 2g, the G′ values were higher than the G′′ in the whole frequency range, which indicated that the formed hydrogels exhibited a significantly elastic behavior. In addition, the G′ values of the ST hydrogel were always higher than that of the SFMA hydrogel. Furthermore, the tensile tests (Fig. S4a) of the two hydrogels were also performed. Fig. 2h shows that the maximum tensile strengths of the SFMA and ST hydrogels were about 22.3 and 40.3 kPa, respectively. The rheological and tensile tests both demonstrated that TA could improve the mechanical strength of the ST hydrogel. Next, a fifty-cycle tensile test was carried out at a strain of 80% (Fig. S4b). A similar hysteresis curve was observed for all cycles, demonstrating that the ST hydrogel had good fatigue resistance.
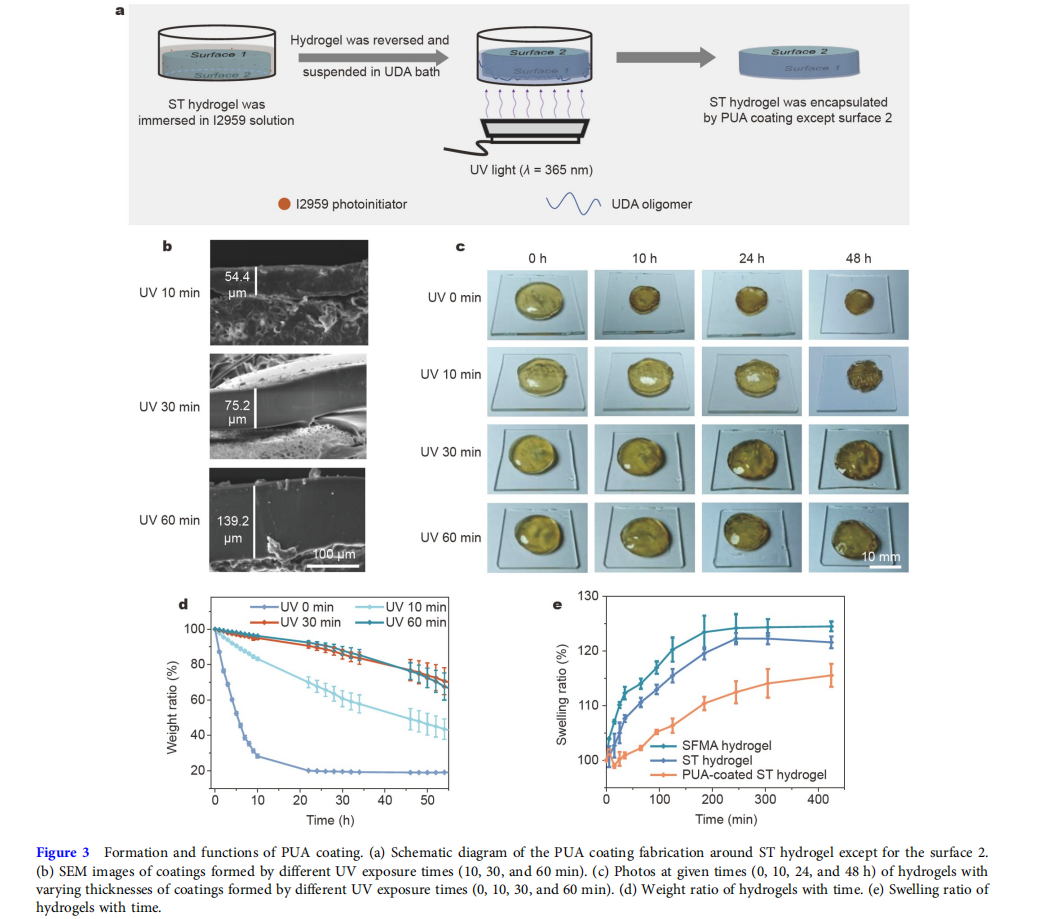
Formation and functions of PUA coating
Fig. 3a schematically shows the formation of the PUA coating on the surfaces of the ST hydrogel. In the first step, the cylindershaped ST hydrogel (diameter = 15 mm, thickness = 2 mm) was placed in a bath, and then immersed in the 0.5 wt% I2959 (a water/oil soluble photoinitiator) solution for 5 min. The hydrogel was taken out and the residual I2959 solution on the surface was wiped off. As shown in Fig. S5, through the reversion procedure, the hydrogel could obtain better water-retention capability, so, in the second step, the hydrogel was reversed and suspended in the oligomeric UDA bath. By exposing the bottom of the UDA bath to the UV light (365 nm), the free-radical polymerization of the UDA oligomer would be triggered by the I2959 photoinitiator to form the PUA coating. Finally, except for the surface 2, the hydrogel was encapsulated by a transparent and shape-fitted PUA coating.
Scanning electron microscopy (SEM) images (Fig. 3b) indicated that by extending the polymerization time from 10 to 60 min, the thickness of PUA coating increased from about 54.4 to 139.2 μm. Next, the water retention capability of hydrogels with varying thicknesses of coatings was tested (Fig. 3c, d). The hydrogel without coating noticeably shrank, and the weight ratio of it was only 28.3 ± 1.1 wt% at 10 h due to the quick water evaporation, and then reached equilibrium (19.9 ± 0.4 wt%) at about 24 h. In comparison, the water retention capability of hydrogels with coatings significantly increased. When the polymerization time of PUA coating was 10 min, the hydrogel could maintain its shape till 24 h, but it would shrink obviously at 48 h, and the weight ratio was only 48.0 ± 6.0 wt%. When the polymerization time was prolonged to 30 and 60 min, the size of corresponding hydrogels showed almost no change at 48 h. Besides, there was no noticeable difference between the weight ratios of the two hydrogels. Based on the water retention capability and polymerization efficiency, we selected the 30 min polymerization time for all subsequent studies, and the ST hydrogel encapsulated by the coating was named PUA-coated ST hydrogel. The low swelling ratio property was also essential to hydrogel dressings because it could decrease the weak mechanical toughness caused by absorbing wound exudate [41,42]. As shown in Fig. 3e, due to the interactions between SFMA chains and TA molecules, the ST hydrogel had a lower swelling ratio than the SFMA hydrogel. The existence of PUA coating could further decrease the swelling ratio by encapsulating the ST hydrogel. In summary, because of the good water retention capability and low swelling ratio, the PUA-coated ST hydrogel could be a hydrogel dressing for wound healing.
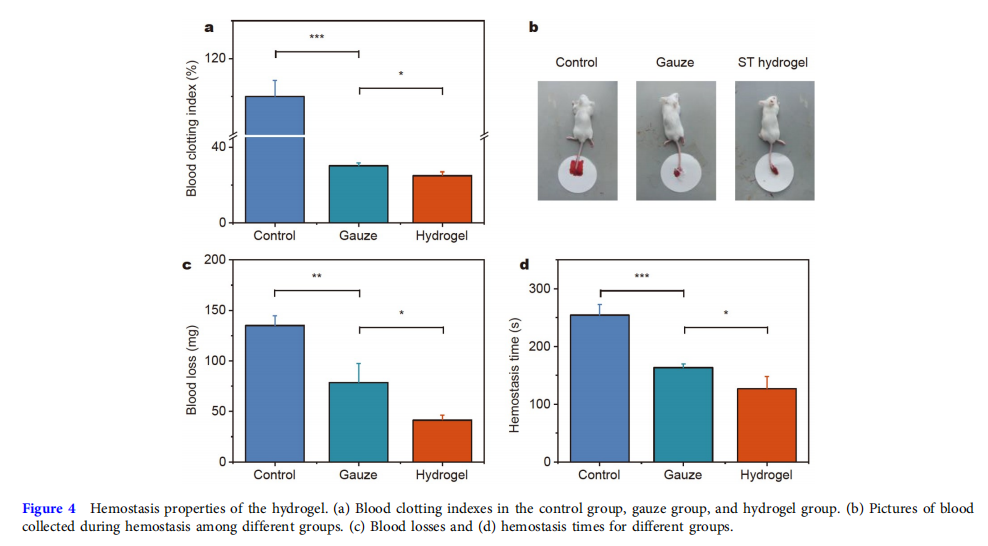
Hemostasis properties of the hydrogel
Generally, hydrogel’s good tissue adhesive property will give it good hemostatic properties [43]. The in vitro hemostatic property of the hydrogel was evaluated by the blood coagulation index (BCI), where the higher BCI value showed a lower clotting rate. Fig. 4a shows the control and gauze groups had higher BCI values than the hydrogel group. Next, the tail amputation model of mice was further established to evaluate the in vivo hemostatic property of the hydrogel (Fig. 4b). As shown in Fig. 4c, the blood loss from the control group (135.0 ± 9.5 mg) was the highest. After being treated with gauze and hydrogel, the blood loss reduced to 78.7 ± 18.7 and 41.3 ± 5.0 mg. Fig. 4d shows that the hemostasis time of the adhesive hydrogel group (126.7 ± 21.4 s) was distinctly shorter than that of the blank (254.3 ± 18.4 s) and gauze (163.3 ± 6.5 s) groups. Gauze had hemostasis properties by absorbing blood plasma to concentrate red blood cells (RBCs) and coagulation factors [44,45]. On the one hand, the hydrogel could stick to the blood area and act as a physical barrier to prevent blood loss. On the other hand, the hydrogen bonding interactions between TA and proteins in blood might also decrease bleeding [16]. Furthermore, SF could induce the formation of thrombin to accelerate blood coagulation [36]. Based on the above data, this adhesive hydrogel had good hemostatic properties.
Biocompatibility of the hydrogel
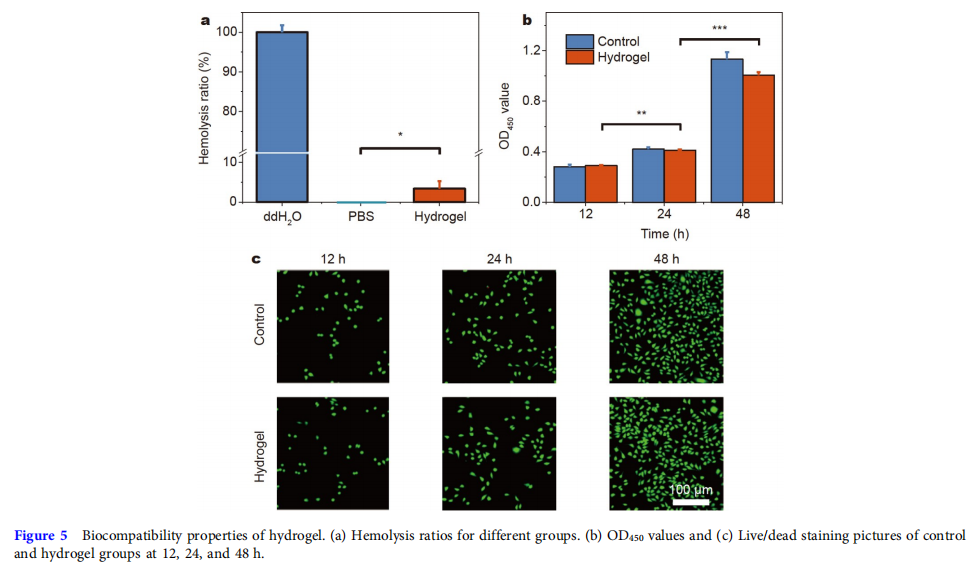
Good biocompatibility was a precondition for wound-healing biomaterials. The rupture of RBCs would lead to the release of platelet, hemoglobin, and even thrombus formation, so the RBCs compatibility was of great importance to wound dressing in contact with blood [46]. A hemolytic activity assay was used to evaluate the hemolysis ratio of the hydrogel. Fig. 5a shows that the hemolysis ratio of the hydrogel was considerably lower than that of the ddH2O group. Besides, the cytocompatibility test of the hydrogel with L929 cells was evaluated. As shown in Fig. 5b, the optical density at 450 nm (OD450) indicated that over 85% of cells of the hydrogel group remained survival at 12, 24, and 48 h, which was much higher than the lowest acceptable standard of nontoxicity of biomaterials (70%) [47,48]. The confocal images of live/dead cell staining (Fig. 5c) indicated that the density of live cells (green fluorescence) of the control and hydrogel groups continuously increased with culturing, and almost no dead cells (red fluorescence) were found. Overall, these hemocompatibility and cytocompatibility results suggest the good biocompatibility of the hydrogel.
Acceleration of PUA-coated ST hydrogel for wound healing
Before the animal experiments, the thickness of the residual adhesive after ten dressing changes was tested. Fig. S6 shows that the thicknesses of the residual adhesives with no patch change and ten patch changes were almost the same. This indicated that the residual adhesive contact on the skin would not be thicker with dressing changes. To assess the practical therapeutic effect of the PUA-coated ST hydrogel, in vivo full-thickness skin defects (diameter = 10 mm) were created on the back of SD rats. These rats were randomly divided into five groups: blank, gauze, ST hydrogel, PUA-coated PST hydrogel, and PUA-coated ST hydrogel groups. The wound healing processes of the five groups were recorded on days 0, 3, 6, and 12 with a digital camera (Fig. 6a). To further visually illustrate the difference in wound healing efficiency between these groups, their dynamic healing processes are shown in Fig. 6c. It was clear that three hydrogel groups had higher healing efficiency than the blank and gauze groups mainly because of the moist environment provided by the hydrogels [15,49]. It could also be seen in Fig. 6b, the wound closure rate of the PUA-coated ST hydrogel group on day 12 was 96.9% ± 0.6%, which was higher than that of the PUA-coated PST hydrogel group (92.2% ± 0.6%), ST hydrogel group (89.1% ± 0.7%), gauze group (82.4% ± 1.2%), and the blank group (86.4% ± 1.2%).
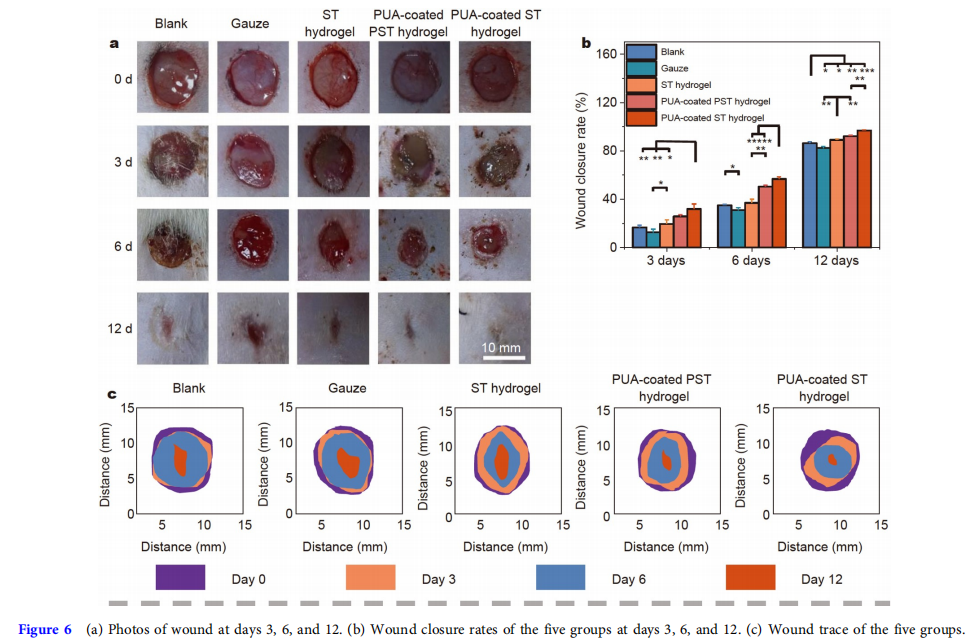
The wound healing efficiency of the gauze group was the lowest because the gauze easily adhered to the wound after absorbing the wound exudate, leading to severe secondary damage to the wound during removal [7,50]. Although ST hydrogel had the advantage of a moist environment in wound healing, this advantage would quickly disappear because ST hydrogel dehydrated rapidly. Owing to the adhesion energy being lower than the fracture energy, the PUA-coated PST hydrogel dressing was inseparable, which would cause damage to the wound during dressing changes. In particular, the PUAcoated ST hydrogel group achieved the highest wound healing efficiency among the three hydrogel groups. On the one hand, the PUA coating endowed the PUA-coated ST hydrogel dressing with a long-lasting water retention capability, allowing for more efficient wound healing by providing a long-lasting moist healing environment. On the other hand, because the adhesion energy was higher than the fracture energy, the PUA-coated ST hydrogel was separable, and residual hydrogel still adhered to the wound, so there was almost no damage to the wound during dressing changes. In conclusion, the wound healing efficiency of the PUA-coated ST hydrogel group was the highest among the five groups.
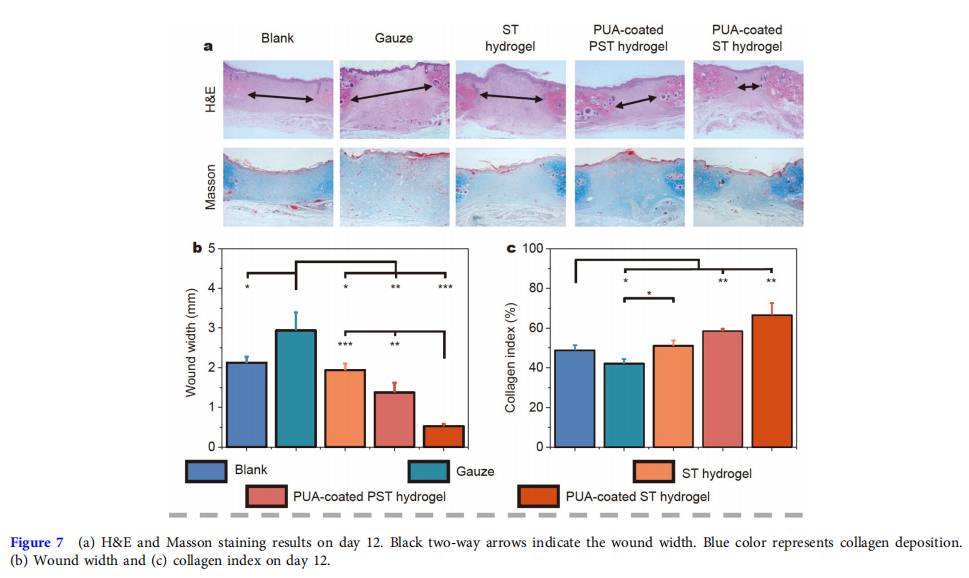
The quality of the wound healing in the five groups on day 12 was further evaluated by hematoxylin and eosin (H&E) and Masson staining (Fig. 7a). The shorter the wound width, the better tissue healing [7]. Fig. 7b demonstrates that the wound width of the PUA-coated ST hydrogel group (0.558 ± 0.021 mm) was the shortest among all groups, and the figures for the PUAcoated PST hydrogel group, ST hydrogel group, gauze group, and blank group were 1.375 ± 0.239, 1.940 ± 0.164, 2.940 ± 0.453, and 2.126 ± 0.155 mm, respectively. Furthermore, the higher the collagen index, the better collagen deposition in the wound [47]. The collagen index results (Fig. 7c) showed that the hydrogel groups, especially the PUA-coated ST hydrogel group (66.5% ± 6.0%), significantly enhanced the deposition of collagen compared with the PUA-coated PST hydrogel (58.5% ± 1.2%), ST hydrogel (51.0% ± 2.7%), gauze (42.2 ± 2.4%), and blank (48.8% ± 2.6%) groups. These histological data agreed well with the observation on wound healing.
The evaluation of secondary damage
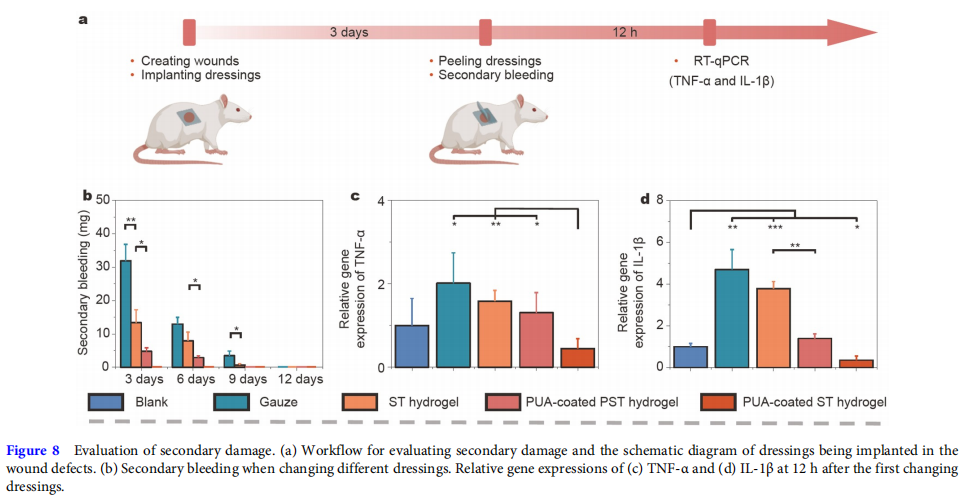
The secondary damage of the five groups was further evaluated to reveal the mechanism of PUA-coated ST hydrogel for accelerating wound healing (Fig. 8a). Because of the re-damage to the wound, secondary damage during dressing changes could be seen as a new injury to the wound and result in secondary bleeding and increased relative gene expression of inflammation factors [22]. Fig. 8b shows that the secondary bleeding of the PUA-coated ST hydrogel group was the lowest at any changing dressings. In addition, the related inflammation factors (TNF-α and IL-1β) markedly increased at about 12 h after a new injury [13,51,52]. Therefore, the relative gene expression of TNF-α and IL-1β at 12 h after the first changing dressings was tested by real time quantitative polymerase chain reaction (RT-qPCR). The primer sequences for RT-qPCR are listed in Table S1. The results (Fig. 8c, d) indicated that the PUA-coated ST hydrogel group had the lowest relative gene expression of the two inflammation factors. These results were most likely due to the long-lasting water retention and separable abilities of the PUA-coated ST hydrogel.
CONCLUSIONS
In this study, we developed a water-retaining and separable adhesive PUA-coated ST hydrogel wound dressing with good hemostatic properties and biocompatibility. The evaluation of adhesion energy and fracture energy showed that the separable ST hydrogel could be obtained by immersing the SFMA hydrogel in 5 wt% TA solution for 2 h. Next, the hydrophobic PUA coating was formed on the surfaces of the hydrogel to prevent water evaporation. Compared with the blank, gauze, ST hydrogel, and PUA-coated PST hydrogel dressings, the PUAcoated ST hydrogel dressing could improve the wound healing efficiency by not only providing a long-lasting moist environment but also being separated without secondary damage during dressing changes. Besides, it should be noted that the residual adhesive could not be removed, so for wounds that need cleaning, such as infected wounds, this hydrogel dressing might be not suitable. In summary, the PUA-coated ST hydrogel promoted wound healing and brought a new strategy for the next-generation design of adhesive hydrogel wound dressings.
Received 24 December 2022; accepted 31 March 2023;
published online 14 June 2023
References
1 Li Z, Zhou F, Li Z, et al. Hydrogel cross-linked with dynamic covalent bonding and micellization for promoting burn wound healing. ACS Appl Mater Interfaces, 2018, 10: 25194–25202
2 Dimatteo R, Darling NJ, Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliver Rev, 2018, 127: 167–184
3 Hao R, Cui Z, Zhang X, et al. Rational design and preparation of functional hydrogels for skin wound healing. Front Chem, 2021, 9: 839055
4 Zhao X, Liang Y, Huang Y, et al. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv Funct Mater, 2020, 30: 1910748
5 Dai Z, Zhang Y, Chen C, et al. An antifouling and antimicrobial zwitterionic nanocomposite hydrogel dressing for enhanced wound healing. ACS Biomater Sci Eng, 2021, 7: 1621–1630
6. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care, 2015, 4: 560–582
7. Huangfu Y, Li S, Deng L, et al. Skin-adaptable, long-lasting moisture, and temperature-tolerant hydrogel dressings for accelerating burn wound healing without secondary damage. ACS Appl Mater Interfaces, 2021, 13: 59695–59707
8. Li S, Chen A, Chen Y, et al. Lotus leaf inspired antiadhesive and antibacterial gauze for enhanced infected dermal wound regeneration. Chem Eng J, 2020, 402: 126202
9. Junker JPE, Kamel RA, Caterson EJ, et al. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Care, 2013, 2: 348–356
10. Dyson M, Young SR, Hart J, et al. Comparison of the effects of moist and dry conditions on the process of angiogenesis during dermal repair. J Investig Dermatol, 1992, 99: 729–733
11. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature, 1962, 193: 293–294
12. Zhang L, Liu M, Zhang Y, et al. Recent progress of highly adhesive hydrogels as wound dressings. Biomacromolecules, 2020, 21: 3966–3983
13. Liang Y, He J, Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano, 2021, 15: 12687–12722
14. Pan G, Li F, He S, et al. Mussel- and barnacle cement proteins-inspired dual-bionic bioadhesive with repeatable wet-tissue adhesion, multimodal self-healing, and antibacterial capability for nonpressing hemostasis and promoted wound healing. Adv Funct Mater, 2022, 32: 2200908
15. Cao C, Yang N, Zhao Y, et al. Biodegradable hydrogel with thermoresponse and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today, 2021, 39: 101165
16. Xu G, Xu N, Ren T, et al. Multifunctional chitosan/silver/tannic acid cryogels for hemostasis and wound healing. Int J Biol Macromolecules, 2022, 208: 760–771
17. He X, Liu X, Yang J, et al. Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing. Carbohydrate Polyms, 2020, 247: 116689
18. Fan H, Wang J, Zhang Q, et al. Tannic acid-based multifunctional hydrogels with facile adjustable adhesion and cohesion contributed by polyphenol supramolecular chemistry. ACS Omega, 2017, 2: 6668–6676
19. Wang B, Xin T, Shen L, et al. Acoustic transmitted electrospun fibrous membranes for tympanic membrane regeneration. Chem Eng J, 2021, 419: 129536
20. Liu H, Qin S, Liu J, et al. Bio-inspired self-hydrophobized sericin adhesive with tough underwater adhesion enables wound healing and fluid leakage sealing. Adv Funct Mater, 2022, 32: 2201108
21. Liu B, Wang Y, Miao Y, et al. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and Ekin. Biomaterials, 2018, 171: 83–96
22. Guo B, Dong R, Liang Y, et al. Haemostatic materials for wound healing applications. Nat Rev Chem, 2021, 5: 773–791
23. Zhou Z, Xiao J, Guan S, et al. A hydrogen-bonded antibacterial curdlan-tannic acid hydrogel with an antioxidant and hemostatic function for wound healing. Carbohydr Polym, 2022, 285: 119235
24. Sekine Y, Ikeda-Fukazawa T. Structural changes of water in a hydrogel during dehydration. J Chem Phys, 2009, 130: 034501
25. Wu J, Wu Z, Xu H, et al. An intrinsically stretchable humidity sensor based on anti-drying, self-healing and transparent organohydrogels. Mater Horiz, 2019, 6: 595–603
26. Ying B, Liu X. Skin-like hydrogel devices for wearable sensing, soft robotics and beyond. iScience, 2021, 24: 103174
27. Subraveti SN, Raghavan SR. A simple way to synthesize a protective “skin” around any hydrogel. ACS Appl Mater Interfaces, 2021, 13: 37645–37654
28. Zhu T, Jiang C, Wang M, et al. Skin-inspired double-hydrophobiccoating encapsulated hydrogels with enhanced water retention capacity. Adv Funct Mater, 2021, 31: 2102433
29. Mredha MTI, Le HH, Cui J, et al. Double-hydrophobic-coating through quenching for hydrogels with strong resistance to both drying and swelling. Adv Sci, 2020, 7: 1903145÷÷
30. Jiang Y, Zhang X, Zhang W, et al. Infant skin friendly adhesive hydrogel patch activated at body temperature for bioelectronics securing and diabetic wound healing. ACS Nano, 2022, 16: 8662–8676
31. Liang Y, Li Z, Huang Y, et al. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano, 2021, 15:7078–7093
32. Kim SH, Hong H, Ajiteru O, et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat Protoc, 2021, 16: 5484–5532
33. Zhang W, Chen L, Chen J, et al. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv Healthcare Mater, 2017, 6: 1700121
34. Bai S, Zhang X, Cai P, et al. A silk-based sealant with tough adhesion for instant hemostasis of bleeding tissues. Nanoscale Horiz, 2019, 4: 1333–1341
35. Kundu B, Rajkhowa R, Kundu SC, et al. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliver Rev, 2013, 65: 457–470
36. Seib FP, Maitz MF, Hu X, et al. Impact of processing parameters on the haemocompatibility of Bombyx mori silk films. Biomaterials, 2012, 33: 1017–1023
37. Guan G, Zhang Q, Jiang Z, et al. Multifunctional silk fibroin methacryloyl microneedle for diabetic wound healing. Small, 2022, 18: 2203064
38. Zhang Q, Qiao Y, Li C, et al. Chitosan/gelatin-tannic acid decorated porous tape suture with multifunctionality for tendon healing. Carbohydrate Polyms, 2021, 268: 118246
39. Yang C, Suo Z. Hydrogel ionotronics. Nat Rev Mater, 2018, 3: 125–142
40. Zhang X, Chen G, Wang Y, et al. Arrowhead composite microneedle patches with anisotropic surface adhesion for preventing intrauterine adhesions. Adv Sci, 2022, 9: 2104883
41. Xu Q, A S, Gao Y, et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater, 2018, 75: 63–74
42. Zhang L, Zhang Y, Ma F, et al. A low-swelling and toughened adhesive hydrogel with anti-microbial and hemostatic capacities for wound healing. J Mater Chem B, 2022, 10: 915–926
43. Liang Y, Li M, Yang Y, et al. pH/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano,
44. Zhang W, Wu J, Yu L, et al. Paraffin-coated hydrophobic hemostatic zeolite gauze for rapid coagulation with minimal adhesion. ACS Appl Mater Interfaces, 2021, 13: 52174–52180 2022, 16: 3194–3207
45. Xu Y, Fang Y, Ou Y, et al. Zinc metal-organic framework@chitin composite sponge for rapid hemostasis and antibacterial infection. ACS Sustain Chem Eng, 2020, 8: 18915–18925
46. Song X, Wang K, Tang CQ, et al. Design of carrageenan-based heparinmimetic gel beads as self-anticoagulant hemoperfusion adsorbents. Biomacromolecules, 2018, 19: 1966–1978
47. Qian J, Ji L, Xu W, et al. Copper-hydrazide coordinated multifunctional hyaluronan hydrogels for infected wound healing. ACS Appl Mater Interfaces, 2022, 14: 16018–16031
48. Bahadoran M, Shamloo A, Nokoorani YD. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGFencapsulated microspheres for accelerated wound healing. Sci Rep, 2020, 10: 7342
49. Yu R, Zhang H, Guo B. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nano-Micro Lett, 2022, 14: 1
50. Zhang M, Zhao X. Alginate hydrogel dressings for advanced wound management. Int J Biol Macromol, 2020, 162: 1414–1428
51. Hübner G, Brauchle M, Smola H, et al. Differential regulation of proinflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine, 1996, 8: 548–556
52. Sato Y, Ohshima T. The expression of mRNA of proinflammatory cytokines during skin wound healing in mice: A preliminary study for forensic wound age estimation (II). Int J Legal Med, 2000, 113: 140–145
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31971326 and 52203205).
Author contributions
Zhang Z performed the experiments and wrote the original manuscript. Zhang Y and Wang J supervised the experiments and revised the manuscript. Liu Y and Liu X guided the synthesis of hydrogel.
This article is excerpted from the SCIENCE CHINA Materials by Wound World.


