INTRODUCTION
Skin tissue is a natural barrier that protects the human body from harmful substances (viruses, bacteria, etc.), playing an essential role in maintaining the balance of the body’s internal environment [1,2]. However, the treatment of skin wounds has become a major clinical challenge that has created a substantial economic burden worldwide, especially in chronic wounds [3,4]. A classic case of chronic wounds is diabetic wounds, often accompanied by severe bacterial infection and slow healing. Diabetic neuropathy, excessive inflammation, peripheral vascular disease, and wound infection are the principal causes of diabetic wounds, and improper treatment can lead to serious negative consequences such as extensive ulceration and sepsis [5–8]. However, the current research on diabetic wounds mainly focuses on wound infection and angiogenesis, lacking the exploration of immunomodulation and neuroregeneration. Therefore, developing a novel strategy for treating diabetic wounds and simultaneously exploring the process of epidermal nerve repair and macrophage polarization is highly desirable.
Hydrogel is a polymer material with a three-dimensional network structure, which can absorb tissue exudates from the wound, maintain a relatively moist wound environment, and protect the wound from microbial invasion [9–13]. Therefore, hydrogel dressings have been widely used in wound treatment and as an ideal candidate material for diabetic wound healing [14,15]. Polydopamine (PDA)-containing hydrogels have shown great potential in various biomedical applications and received increasing attention due to their excellent biological properties. For example, Guo and co-workers [16] fabricated a multifunctional hydrogel dressing for diabetic foot wounds by introducing the PDA-coated reduced graphene oxide. Wu and co-workers [17] synthesized a near-infrared light-triggerable thermo-sensitive hydrogel based on the PDA nanoparticles and glycol chitosan, which exhibited unusual bacterial killing activity and outstanding wound healing ability. Furthermore, wound repair is a complex process including inflammation, proliferation, and remodeling [18–20]. Nonetheless, most hydrogel dressings only focus on reducing wound infection during the inflammatory stage and rarely participate in endogenous cell behaviors of wound healing as well as the neural repair process, leading to a passive repair process. Thus, there is an urgent need for hydrogel dressings that reduce infection while promoting cell proliferation, angiogenesis, and epidermal nerve repair, which are critical for accelerating the healing of diabetic wounds.
During diabetic wound healing, the endogenous direct current electric field (DCEF) is generated by the difference in transepithelial potential, which is of undoubted importance in regulating cell behavior to promote wound healing and regeneration [21–23]. Inspired by the endogenous EF, exogenous electrical stimulation (ES) at the wound site provides an effective strategy for accelerating wound healing. At present, the research reports have confirmed that exogenous ES can accelerate wound healing by enhancing the proliferation and migration of fibroblasts and promoting the secretion of more extracellular matrix by keratinocytes [24–28]. However, exogenous ES needs to supply current by implanting electrodes near the wound surface and not covering the entire wound surface, which seriously affects the therapeutic effect of exogenous ES. To give full play to the role of ES, the synergistic therapeutic strategy combining hydrogel with ES provides a new idea for accelerating the healing of diabetic wounds.
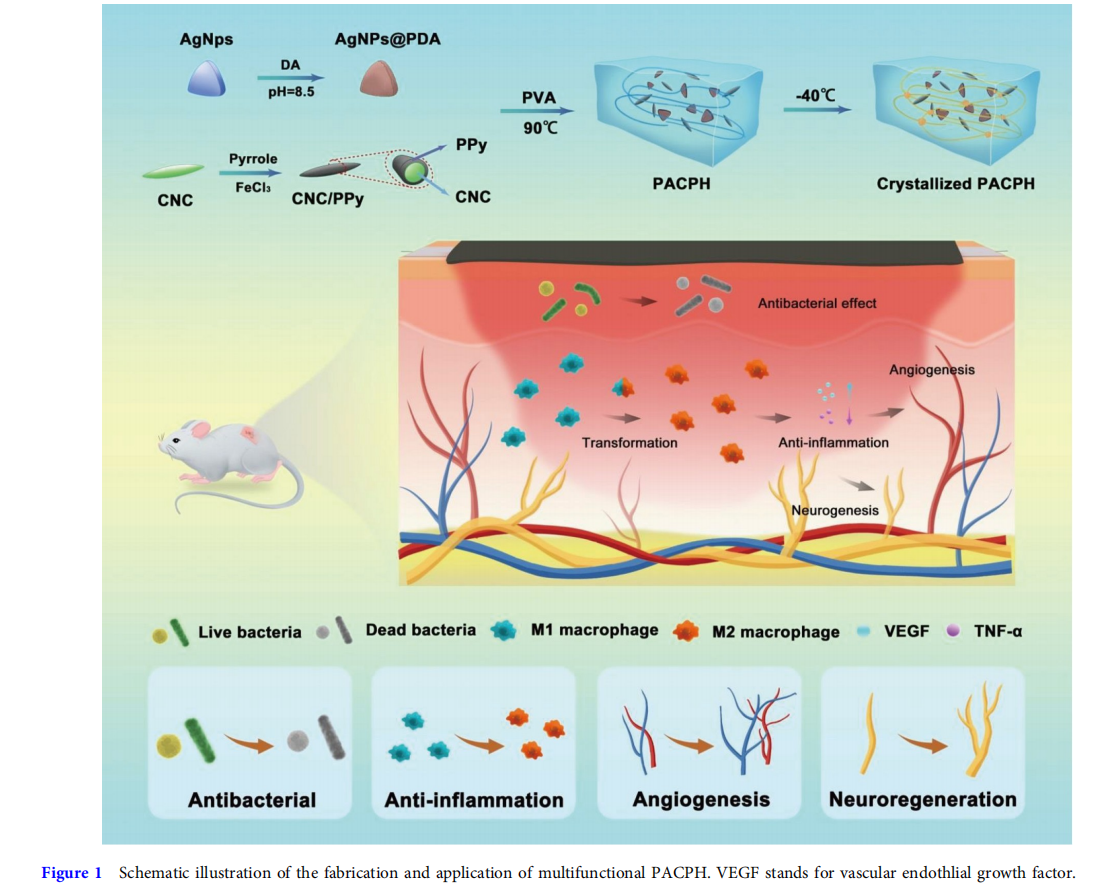
Herein, we demonstrate a novel strategy to fabricate a kind of multifunctional hydrogel dressing. The hydrogel dressing, abbreviated as PACPH (comprising PDA, Ag nanoparticles (AgNPs), cellulose nanocrystals (CNC), polypyrrole (PPy), and polyvinyl alcohol (PVA)), has exceptional mechanical properties, excellent adhesive and photothermal performance, broad antibacterial activity, and outstanding electroactivity. Among them, the PDA-modified silver nanoparticles (AgNPs@PDA) as antibacterial units of the PACPH exhibit favorable antibacterial activity against both Gram-positive (S. aureus) and Gramnegative (E. coli) bacteria. Meanwhile, the free-catechol groups on PDA have a good interfacial binding affinity with nucleophiles (i.e., –NH2 and –SH groups) on the surface of biological tissues, providing excellent adhesion for hydrogel dressings. A composite of CNC/PPy as the conductive matrix endows the hydrogel with desirable electrical activity, which provides the possibility for the therapeutic strategy of combining the hydrogel with exogenous ES. We found that the strategy of hydrogel combined with ES has a significant therapeutic effect on diabetic wounds by inhibiting inflammation, and promoting macrophage polarization, angiogenesis, and nerve repair. To the best of our knowledge, this is the first time that combining conductive hydrogel with ES has a significant promoting effect on the nerve repair of diabetic wounds. Therefore, this strategy provides an effective way for diabetic chronic wound management and would stimulate more new approaches to construct new-type wound dressings.
RESULTS AND DISCUSSION
Formation mechanism of the PACPH
The synthesis process of the PACPH and its application in diabetic wound dressing are schematically illustrated in Fig. 1. CNC is a renewable biomass material with good biocompatibility, high specific surface area, and surface hydroxyl modification, making it a good choice as a stable nanocarrier [29–33]. In this work, by using CNC as a biological template, PPy coating was grown on the surface of CNC by in situ polymerization of pyrrole monomers to obtain CNC/PPy complexes. The CNC/PPy complexes have outstanding water dispersibility compared with the pure PPy after standing for 24 h (Fig. S1). From contact angle measurements, the CNC/PPy complex film also exhibited favorable hydrophilicity, mainly due to the strong hydrogen bonding between PPy and CNC (Fig. S2). CNC/PPy complexes were characterized by Fourier transform infrared spectroscopy (FTIR) and UV-vis absorption spectroscopy (UV), and the results further verified that PPy was successfully modified on the surface of CNC (Figs S3 and S4). Notably, the introduction of CNC/PPy complexes endowed the hydrogel with excellent electroactivity. Compared with the PAPH, the conductivity of PACPH was significantly improved and the conductivity was gradually enhanced with the increase of CNC/PPy content (Figs S5 and S6). Subsequently, AgNPs@PDA were introduced into the hydrogel system, which endowed the hydrogel with excellent antibacterial activity, conductivity, and adhesion. And the AgNPs with triangular shapes were characterized with transmission electron microscopy (TEM) in Fig.S7. Further PVA and CNC/PPy complexes were added into the AgNPs@PDA solution to complete dissolution which was then poured into molds and frozen at −40°C overnight to obtain the PACPH (Fig. S8). The obtained hydrogel with desired mechanical properties, favorable adhesion, and broad antibacterial activity is a potential candidate for wound dressings. Moreover, the functional hydrogel combining with ES can effectively accelerate wound healing, which has a significant therapeutic effect on diabetic wounds.
Mechanical property
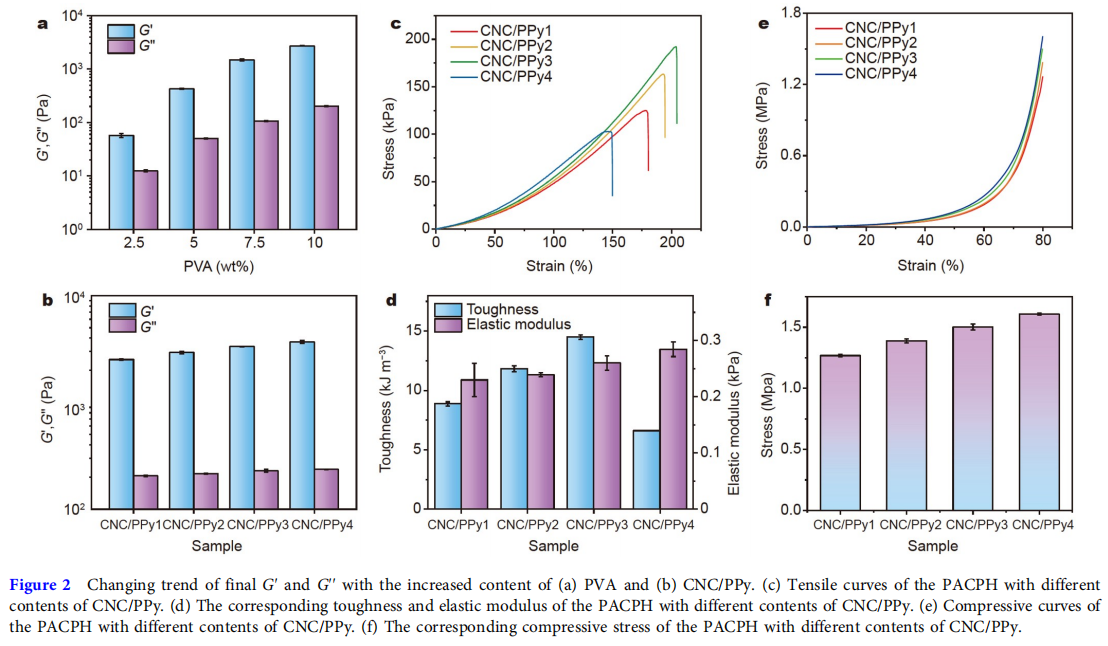
As the largest tissue organ of the human body, the skin will undergo various deformations during human activities and can protect the human body from external damage. Therefore, good mechanical properties are the key to ensuring the long-term use of skin wound dressings, which should have similar mechanical properties to human skin, protecting the wound from secondary damage. The PACPH exhibited extraordinary mechanical behaviors. As illustrated in Fig. 2a, b, the content of PVA and CNC/PPy had a strong influence on the mechanical performance of PACPH. With the increase of PVA content, the storage modulus (G′) and loss modulus (G′′) increased gradually. It is mainly attributed to the crystallization of more PVA chains, increasing the crosslinking density of the hydrogel. In addition, the increase of CNC/PPy concentration also played a role in enhancing the mechanical properties of the PACPH. It was found that the G′ and G′′ of the PACPH increased as CNC/PPy concentration increased, which was consistent with the tensile curves and the corresponding elastic modulus as shown in Fig. 2c, d. The introduction of CNC/PPy effectively improved the mechanical properties of the PACPH due to the gradual enhancement of the hydrogen bonding between CNC, Ppy, and PVA. Further increase of the CNC/PPy concentration resulted in a decline of the tensile strength (CNC/PPy4) but an increase of elastic modulus, which was mainly due to the excessive rigidity of the network. The PACPH hydrogel with CNC/Ppy3 component exhibited the best mechanical properties, with tensile stress, strain, and toughness reaching up to 192 kPa, 200%, and 14.5 kJ m−3 , respectively. Based on the above results, the PACPH hydrogel with CNC/PPy3 component was used as the optimal component in this study and applied in the subsequent in vivo experiment. Subsequently, the compressive performance of the PACPH was investigated at a strain of 80%. The compressive stress of the PACPH also gradually increased with the increase of CNC/PPy concentration, which was consistent with the above tensile results, and the compressive curves and the corresponding compressive stress were displayed in Fig. 2e, f. The outstanding mechanical properties of the PACPH were also demonstrated by loading, stretching, knotted stretching, twisted stretching, and compressing (Fig. S9). It is well known that the stretchability of human skin is 20%–180%. Therefore, the excellent deformability of PACPH can meet the needs of artificial skin and withstand external or local compression, which has great application potential as wound healing dressings.
Adhesion property and photothermal performance
The adhesion and photothermal performance of the PACPH were displayed in Fig. 3. We first explored the adhesive properties of PACPH by observing the oxidation process of DA. As shown in the inset of Fig. 3a, the AgNPs solution appeared blue before DA oxidation, and gradually turned dark brown after 2 h oxidation, suggesting the synthesis of AgNPs@PDA. Moreover, the UV-vis absorption spectra exhibited the characteristic peak of AgNPs appearing at 630 nm (oxidation 0 min). With the increase of DA oxidation time, the characteristic peak of AgNPs disappeared, while the characteristic peak intensity of the benzene group gradually increased at 280 nm (Fig. 3a), indicating the formation of the oxidized o-quinone groups and the complete coating of AgNPs by PDA. Lap-shear measurements were performed to estimate the adhesion performance of PACPH. The adhesive strength of PACPH was significantly affected by the content of DA (Fig. 3b). When the content of DA was 1.5 mmol L−1 , the maximum adhesive strength of PACPH reached 25.95 kPa on the surface of Ti, which was mainly due to the increase of the catechol groups. And this content of DA was selected for the remaining tests. As displayed in Fig. 3c, the adhesion performance of PACPH on different surfaces was also investigated. The maximum adhesive strengths on various surfaces including Ti, Al, Cu, Glass, polymethyl methacrylate (PMMA), and acrylonitrile butadiene styrene (ABS) were 25.95, 22.17, 20.41, 19.66, 18.92, and 17.2 kPa, respectively. The demonstrations of PACPH adhering to various materials were illustrated in Fig. S10a. The PACPH can firmly adhere to diverse organic and inorganic material substrates. The results demonstrated that PDA played a key role in enhancing the adhesion of the PACPH. The catechol groups on PDA chains can interact with diverse inorganic substrates via hydrogen bonding, metal complexation, π-π stacking, and electrostatic effects. Meanwhile, the catechol converts into quinone quickly upon oxidation, which can form covalent bonds with amino or thiol groups in the organic substrates, endowing the PACPH with exceptional tissue adhesion. Additionally, the PACPH can also adhere closely to the human skin and be removed without any residue or allergic reaction (Fig. S10b), suggesting broad potential applications on wound dressings.
To investigate the photothermal performance of the PACPH, the temperature changes were monitored under laser irradiation (808 nm, 1 W cm−2 ). As shown in Fig. 3d, the temperature change of the blank hydrogel group, the PAPH group containing only PDA, the CPH group containing only PPy, and the PACPH group finally reached 8.1, 12.2, 15.9, and 25.2°C within 10 min, and the PACPH had the best photothermal conversion efficiency, demonstrating that the addition of PDA and PPy endowed the hydrogels with outstanding photothermal performance. The photothermal efficacy of PACPH under different laser power densities was displayed in Fig. 3e, showing that the photothermal performance of PACPH was promoted along with the increase of the power densities of the laser irradiation. Furthermore, the PACPH still kept great photothermal performance after four cycles of laser “on-off” (Fig. 3f). As shown in Fig. 3g, the temperature change of hydrogels was observed under the laser irradiation by using an infrared thermal camera, further confirming the stable and terrific photothermal converting ability of the PACPH.

Antibacterial activity assay
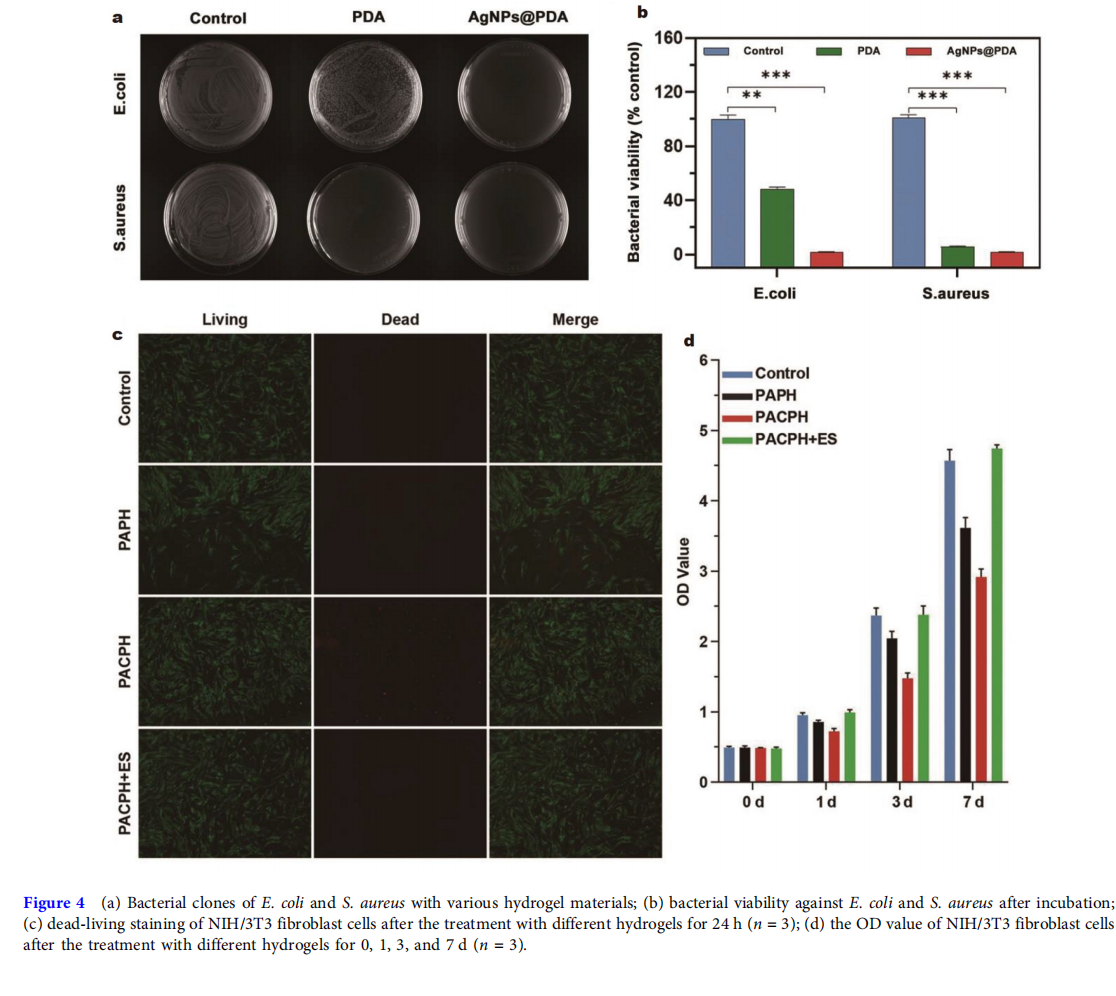
The wound’s bacterial infection is a major factor in the difficulty of diabetic wound healing [34]. Therefore, hydrogel dressings with antibacterial ability are of great value in the clinical treatment of diabetic wounds. The antibacterial activity of the hydrogel dressing against E. coli (Gram-negative bacteria) and S. aureus (Gram-positive bacteria) was evaluated by calculating viable colonies with the standard spread plate method. As shown in Fig. 4a, b, the bacterial activity in the culture dishes treated with the PCPH group containing only PDA and the PACPH group containing AgNPs@PDA was significantly reduced compared with the blank group, indicating that hydrogels had a significant inhibitory effect on bacterial proliferation whether in gram-positive or -negative bacteria. The key reason was that PDA and AgNPs can cause bacterial cell membrane disruption and leakage of cytoplasmic contents [35,36]. Additionally, the culture dishes treated with the PACPH group exhibited substantially lower bacterial activity than the PCPH group, wherein almost 100% of the bacterial colonies were reduced, mainly due to the synergistic antibacterial effect of PDA with AgNPs. Thus, the effective antibacterial properties endow PACPH with the pivotal capacity to prevent wound infections during the diabetic wound repair process.
Biocompatibility and toxicity tests
Good biocompatibility of biomaterials is the key to maintaining cell viability and proliferation. Cell proliferation during wound healing is mostly associated with the proliferation of fibroblasts, and therefore the proliferation test of NIH/3T3 fibroblasts can be used to measure the biocompatibility of the hydrogel dressings. The dead-living cell staining of all hydrogel groups was shown in Fig. 4c. Most cells in the hydrogel groups survived (green) and had a spindle-shaped morphology, suggesting that all the groups had good cytocompatibility. After 1, 3, and 7 days of hydrogel culture, the optical density (OD) values of cells in each group gradually increased, further proving the cell proliferation capacity of PAPH, PACPH, and PACPH+ES groups (Fig. 4d). In addition, there were significantly more cells in the PACPH+ES group than in the other groups, indicating that ES can effectively promote cell proliferation. Based on the above results, the strategy of conductive hydrogel combining with ES provides a new idea for the treatment of chronic wounds.
Diabetic wound healing assessment and histological analysis
To study the therapeutic effect of hydrogel combined with ES on diabetic wound repair, we established a model of type II diabetes mellitus (TIIDM) rats caused by a high-fat diet and streptozotocin. The full-thickness skin defect model was used to estimate the induction and promotion of diabetic rat wound healing. The diabetic wound repair experiments involved the control group (no treatment) and experimental groups (PAPH, PACPH, and PACPH+ES). The residual area of the wound was the first index of wound healing. The optical images of the wounds in the control group and the experimental groups on day 0, 3, 6, 9, 12, and 15 of treatment were shown in Fig. S11a. Compared with the control group, the new epithelium formed earlier in the experimental groups (PAPH, PACPH, and PACPH+ES). The rate of wound healing was faster in the PAPH group than in the PACPH group during the first 9 days of wound healing, mainly related to the fact that the diabetic wounds were mainly in the inflammatory phase, and the promotion of endogenous currents was weaker during the inflammatory phase. The trend of rapid healing in the wounds of the PACPH group during 9–12 days was mainly related to the pro-healing effect of changes in endogenous currents induced by the conductive materials on the wounds mainly during the late inflammatory and proliferation phases. The wound healing effect of the PACPH+ES group was more significant than those of the other hydrogel single-treatment groups. Fig. S11b exhibited the ratio of wound residual area at 0, 3, 6, 9, 12, and 15 days after treatment with PAPH, PACPH, and PACPH+ES. At each observation time, the wound area of the treatment groups (PAPH, PACPH, and PACPH+ES) was smaller than the control group. On day 15, the wound residual area ratio of the PACPH group (12.33%) was lower than the PAPH group (19%), demonstrating that the electroactive component of the hydrogel was beneficial to skin repair in diabetic wounds. Surprisingly, the wound of the PACPH+ES group healed completely, and the wound healing effect was significantly better than the other groups. The wound residual area ratio of the PACPH+ES group (2.67%) was obviously lower than the PACPH group (12.33%) on day 15, illustrating that the therapeutic regimen of the antibacterial conductive hydrogel combined with ES was effective and can further facilitate the wound healing of diabetic wounds. This is mainly due to the ES could maintain or enhance the endogenous current in the wound environment, promoting fibroblast migration, angiogenesis, and epidermal nerve repair, and we will further verify the experimental results next.
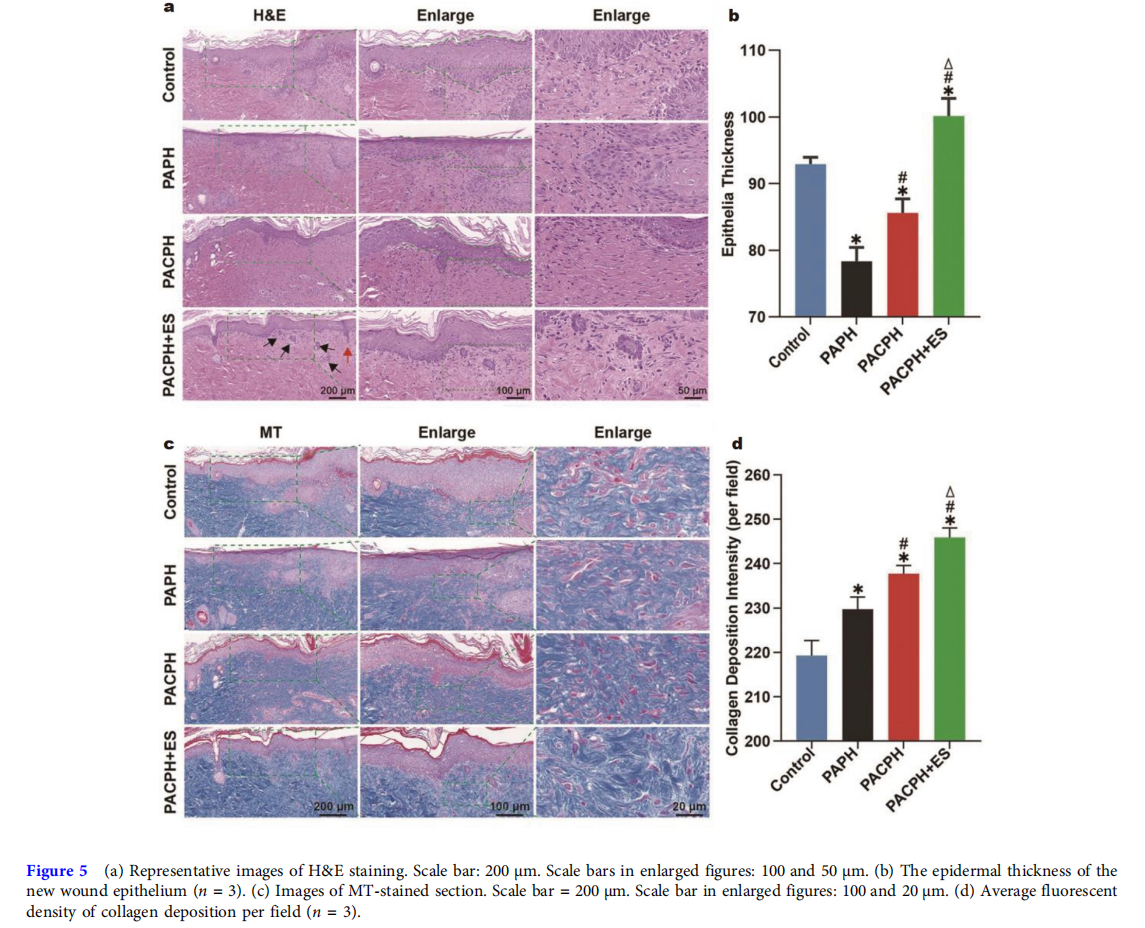
To evaluate the healing effect of the hydrogel dressing groups in histological view, hematoxylin and eosin (H&E) and Masson trichrome (MT) staining were performed on the regenerated skin tissues which were collected on day 15. As shown in Fig. 5a, keratinocytes migrated to the site of the wound and formed a new epithelial layer at the wound’s edge. The control group’s wound contracture was less apparent compared with the other groups. The PAPH group exhibited incomplete nascent epithelium, incomplete epithelialization, and unclear epidermal and dermal borders compared with the PACPH group. In contrast, the formation of a new epithelial stratum corneum and the clear demarcation between the epidermis and dermis of the new epithelium were observed in the PACPH group. Notably, the complete dermis and thick epithelial layer were reconstructed by the group combining PACPH with ES, the dermal and epidermal structures were clearly demarcated, and clear cutaneous appendages such as hair follicles (red arrow) and sebaceous glands (black arrow) were visible in the new epithelium, indicating that the wound has undergone a wound remodeling phase and the new tissue was more mature and closer to the normal tissue. In addition, we further selected the new epithelium at the junction of normal epithelium and new epithelium to quantify the thickness of the new epithelium in Fig. 5b. The epithelial thickness of the control group was higher than those of the PAPH group and PACPH group, mainly due to the epithelial swelling caused by the heavier inflammation in the control group. Meanwhile, the epithelial thickness was significantly increased in the PACPH+ES group compared with the other groups, which had the best healing. Collagen is the primary component of the dermal extracellular matrix (ECM), which plays a primary role in ECM reorganization and tissue remodeling [37,38]. As illustrated in Fig. 5c, d, only a few collagen fibers were detected by MT staining of the control group, while the density of collagen deposition in the experimental groups gradually increased. The density of collagen deposition had the same trend as described above for the wound healing process. The PACPH+ES group displayed the highest collagen deposition in the wound bed, and the collagen fibers were arranged in a more ordered manner, approaching the normal skin. These results further confirmed that the strategy of combining PACPH with ES had a more effective therapeutic effect on the healing of diabetic wounds.
Immunoregulation
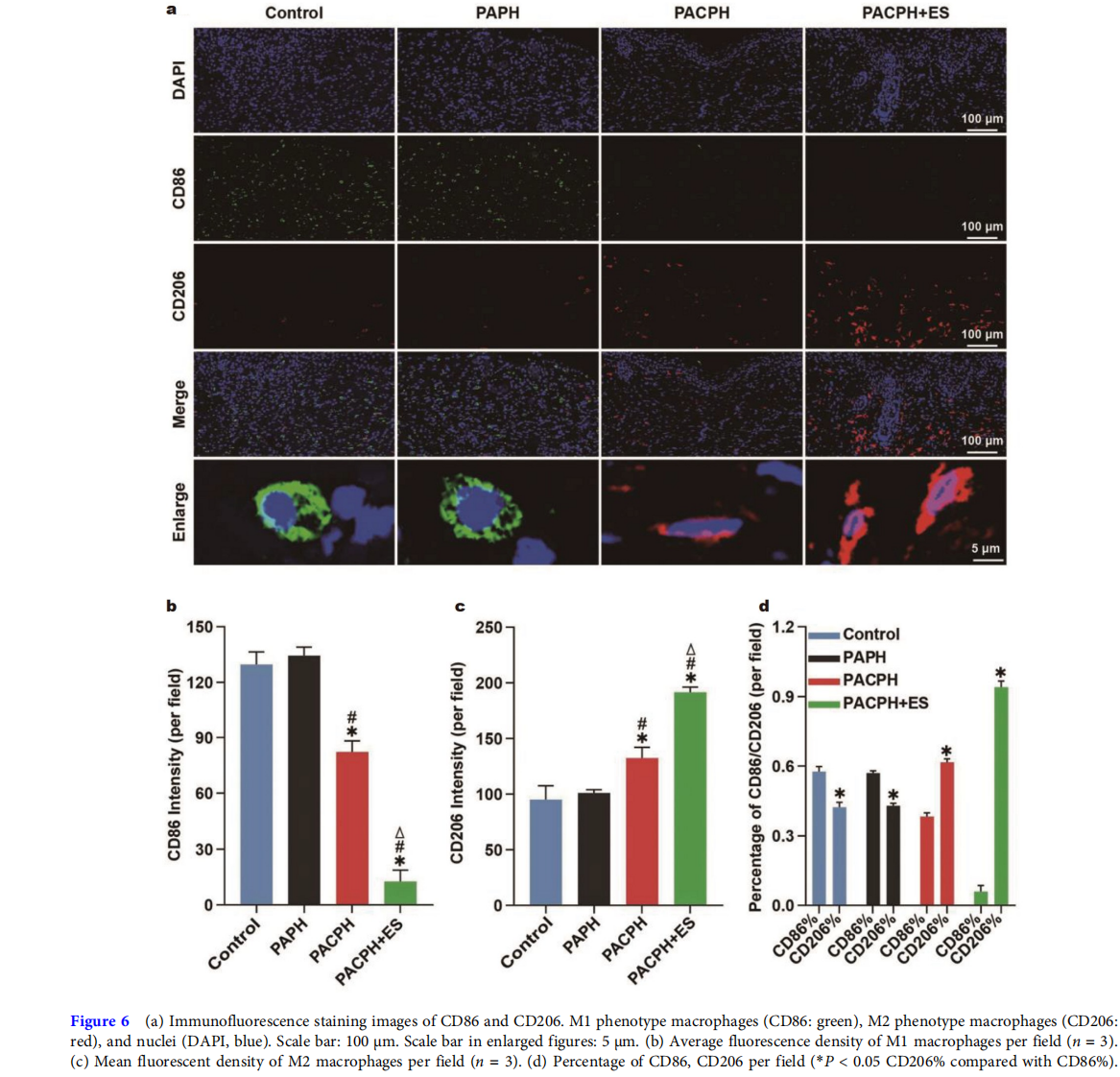
Wound healing is a complex process in which the inflammatory microenvironment plays a crucial role in wound healing and tissue regeneration [39]. Diabetic wounds are chronically inflammatory due to the local microenvironment, which dramatically affects the wound repair of diabetic wounds [40,41]. Macrophages are vital immune cells that regulate inflammation, and can polarize into pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes. In the initial phase of wound healing, the activation of M1 macrophages has the effect of killing pathogens and debriding wounds, while the activation of M2 macrophages mainly contributes to cell proliferation, angiogenesis, and other activities in the second stage of wound healing [42,43]. However, due to the damage of the M2 macrophage polarization process, the inflammation of diabetic wounds is caused, making angiogenesis difficult, which greatly delays the healing of diabetic wounds [44,45]. Hence, M1 and M2 macrophages and their polarization were measured by CD86/CD206 immunofluorescence staining to reveal the anti-inflammatory effect of each group. Among them, CD86 and CD206 are markers of M1 and M2 phenotypes, respectively. As displayed in Fig. 6a, CD86 (green) was predominant, while CD206 (red) was very rare in the control group and PAPH group, suggesting no macrophage phenotypic transformation in the diabetic wound. Moreover, CD206 was significantly upregulated in the PACPH group, and the CD86 expression was the lowest and CD206 expression was the highest in the PACPH+ES group, which consisted primarily of M2 macrophages (Fig. 6b). Meanwhile, the immunofluorescence staining of macrophages treated with the groups of PACPH and PACPG+ES showed more stretched and elongated shapes with pseudopodia-like structures as compared with PAPH and control groups, confirming that the strategy of PACPH combing with ES can effectively regulate the recruitment of macrophages by promoting the transformation of the M1 phenotype to the M2 phenotype, thereby avoiding longterm non-healing of M1 macrophage-induced diabetic wounds.
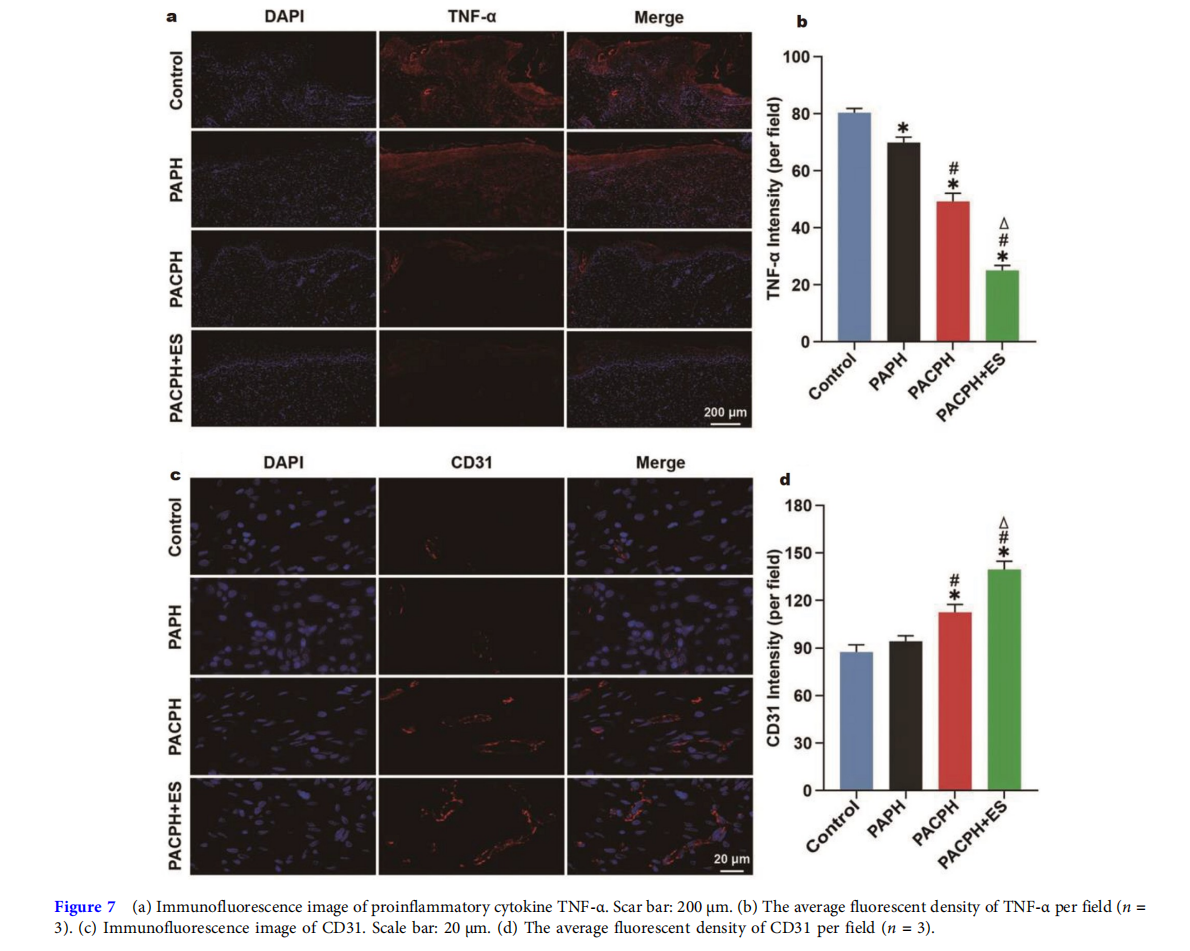
In addition, tumor necrosis factor-alpha (TNF-α) is an essential factor of proinflammatory cytokines, accelerating fibroblast apoptosis and delaying wound healing [42,46]. Therefore, the effects of hydrogel dressings and ES on diabetic wound inflammation were evaluated by TNF-α immunofluorescence staining. As shown in Fig. 7a, b, the level of proinflammatory cytokines in the experimental groups was significantly lower than that in the control group. And the expression of TNF-α in the PACPH+ES group decreased the most, further indicating that the strategy of conductive hydrogel coupling with ES could effectively control the local inflammation of diabetic wounds, improve the local microenvironment, and facilitate diabetic wound healing.
Evaluation of epidermal nerve repair and angiogenesis
Angiogenesis is pivotal to diabetic wound repair [39,47]. Neovascularization can not only attract macrophages and other monocytes to the wound site, but also provide necessary substances for the wound, such as oxygen, nutrients, and growth factors, to accelerate wound healing [47,48]. CD31 is considered an endothelial cell marker for the formation of new capillaries. In this study, the angiogenesis of diabetic wounds was evaluated by CD31 immunofluorescence staining in Fig. 7c, d. The mean fluorescence density of CD31 was not considerably different between the control group and the PAPH group. The blood vessels in the PACPH group were more than in the PAPH group, and the lumen structure was more apparent. Compared with the other groups, the PACPH+ES group had obvious progression of neovascularization with vascular branches, indicating a more excellent blood supply to the wound region, which was more conducive to vascular regeneration.
Moreover, diabetic peripheral neuropathy is a common complication of diabetes, and cutaneous neuropathy occurs earlier and is more evident [40,42]. It is also one of the prominent reasons for the difficulties of diabetic wound healing. Protein gene product-9.5 (PGA-9.5) is a neuronal marker and myelin basic protein (MBP) is the main protein component of the central nervous system and myelin sheath of some neurons. Hence, the effect of conductive hydrogel and ES on nerve repair was assessed by MBP and PGP-9.5 immunofluorescent staining on diabetic wounds (Fig. 8). The phenomenon of epidermal and dermal nerve repair in the control group and PAPH group was a marked lack. By contrast, in the conductive hydrogel PACPH group, moderate PGP-9.5 positive nerve fiber bundles were seen near the epidermal-dermal junction, and a small amount of MBP-positive myelinated nerve fiber bundles were observed in the dermis, confirming that conductive hydrogel had a promoting effect on neuroregeneration in diabetic wounds. Additionally, the PACPH+ES group had the highest positive rate, the nerve fiber bundles at the epidermal-dermis junction extended to the dermis and branched to the skin surface, and the myelinated nerve fiber bundles showed an upward trend in the dermis, disclosing that PACPH coupling with ES had a favorable influence on nerve regeneration, thereby further accelerating the healing of diabetic wounds.

CONCLUSION
In conclusion, the multifunctional PACPH scaffold demonstrated favorable mechanical properties, antibacterial capacity, photothermal performance, tissue adhesion, and outstanding electroactivity through the synergistic integration of AgNPs@PDA and CNC/PPy composites. More importantly, the combination of the functional hydrogel with ES simultaneously exerted various bioactivities, including immunoregulation, angiogenesis, and neuroregeneration, all of which facilitated the favorable wound healing efficiency in the chronic diabetic wound. Overall, this novel strategy for the synergistic treatment of chronic wounds by combining multifunction hydrogel with ES would be of great significance for relevant clinical practice.
Received 5 August 2022; accepted 31 August 2022; published online 18 November 2022
References
1. Clark RAF, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Investig Dermatol, 2007, 127: 1018–1029
2. Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv Drug Deliver Rev, 2019, 146: 344–365
3. Han G, Ceilley R. Chronic wound healing: A review of current management and treatments. Adv Ther, 2017, 34: 599–610
4. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care, 2015, 4: 560–582
5. Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med, 2015, 21: 815–819
6. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet, 2005, 366: 1736–1743
7. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med, 2006, 23: 594–608
8. Patel S, Srivastava S, Singh MR, et al. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother, 2019, 112: 108615
9. Zhao X, Liang Y, Huang Y, et al. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv Funct Mater, 2020, 30: 1910748
10. Qu J, Zhao X, Liang Y, et al. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J, 2019, 362: 548–560
11. Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano, 2019, 13: 10279–10293
12. Zhang J, Zheng Y, Lee J, et al. A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nat Commun, 2021, 12: 1670
13. Zhao Y, Song S, Ren X, et al. Supramolecular adhesive hydrogels for tissue engineering applications. Chem Rev, 2022, 122: 5604–5640
14. Chen J, He J, Yang Y, et al. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater, 2022, 146: 119–130
15. Wang K, Dong R, Tang J, et al. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem Eng J, 2022, 430: 132664
16. Liang Y, Li M, Yang Y, et al. pH/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano, 2022, 16: 3194–3207
17. Gao G, Jiang YW, Jia HR, et al. Near-infrared light-controllable ondemand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials, 2019, 188: 83–95
18. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med, 2014, 6: 265sr6
19. Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature, 2008, 453: 314–321
20. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration,and fibrosis. Immunity, 2016, 44: 450–462
21. Song B, Gu Y, Pu J, et al. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat Protoc, 2007, 2: 1479–1489
22. Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature, 2006, 442: 457–460
23. Wang J, Lin J, Chen L, et al. Endogenous electric-field-coupled electrospun short fiber via collecting wound exudation. Adv Mater, 2022, 34: 2108325
24. Thrivikraman G, Boda SK, Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials, 2018, 150: 60–86
25. Long Y, Wei H, Li J, et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano, 2018, 12: 12533–12540
26. Luo R, Dai J, Zhang J, et al. Accelerated skin wound healing by electrical stimulation. Adv Healthc Mater, 2021, 10: 2100557
27. Yao G, Mo X, Yin C, et al. A programmable and skin temperatureactivated electromechanical synergistic dressing for effective wound healing. Sci Adv, 2022, 8: eabl8379
28. Kai H, Yamauchi T, Ogawa Y, et al. Accelerated wound healing on skin by electrical stimulation with a bioelectric plaster. Adv Healthc Mater, 2017, 6: 1700465
29. Kargarzadeh H, Mariano M, Gopakumar D, et al. Advances in cellulose nanomaterials. Cellulose, 2018, 25: 2151–2189
30. Tang J, Sisler J, Grishkewich N, et al. Functionalization of cellulose nanocrystals for advanced applications. J Colloid Interface Sci, 2017, 494: 397–409
31. Yang X, Biswas SK, Han J, et al. Surface and interface engineering for nanocellulosic advanced materials. Adv Mater, 2021, 33: 2002264
32. Lagerwall JPF, Schütz C, Salajkova M, et al. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater, 2014, 6: e80
33. Shi Z, Phillips GO, Yang G. Nanocellulose electroconductive compoNanoscale, 2013, 5: 3194–3201
34. Wang S, Zheng H, Zhou L, et al. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett, 2020, 20: 5149–5158
35. Zhou L, Zheng H, Liu Z, et al. Conductive antibacterial hemostatic multifunctional scaffolds based on Ti3C2Tx MXene nanosheets for promoting multidrug-resistant bacteria-infected wound healing. ACS Nano, 2021, 15: 2468–2480
36. Mao C, Xiang Y, Liu X, et al. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ ZnO nanostructures. ACS Nano, 2017, 11: 9010–9021
37. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol, 2015, 173: 370–378
38. Cuttle L, Nataatmadja M, Fraser JF, et al. Collagen in the scarless fetal skin wound: Detection with picrosirius-polarization. Wound Repair Regen, 2005, 13: 198–204
39. Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med, 2021, 13: eabe4839
40. Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers. J Am Acad Dermatol, 2014, 70: 1.e1–1.e18
41. Sawaya AP, Stone RC, Brooks SR, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun, 2020, 11: 4678
42. Nowak NC, Menichella DM, Miller R, et al. Cutaneous innervation in impaired diabetic wound healing. Transl Res, 2021, 236: 87–108
43. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol, 2014, 5: 614
44. Nassiri S, Zakeri I, Weingarten MS, et al. Relative expression of proinflammatory and anti-inflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. J Investig Dermatol, 2015, 135: 1700–1703
45. Okizaki S, Ito Y, Hosono K, et al. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed Pharmacother, 2015, 70: 317–325
46. Siqueira MF, Li J, Chehab L, et al. Impaired wound healing in mouse models of diabetes is mediated by TNF-α dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia, 2010, 53: 378–388
47. Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev, 2003, 23: 117–145
48. Qian Z, Wang H, Bai Y, et al. Improving chronic diabetic wound healing through an injectable and self-healing hydrogel with plateletrich plasma release. ACS Appl Mater Interfaces, 2020, 12: 55659–55674
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81901365 and 82003284), the Department of Finance of Jilin Province (2019SRCJ005 and 2020SCZT051), Jilin Science and Technology Agency Funds in China (20210402001GH), the Key Research and Development Project of Jilin Provincial Science and Technology Department (20210204142YY), and the Norman Bethune Program of Jilin University (2022B44).
Author contributions
Guan L designed the research ideas and wrote the article. Ou X performed the animal experiment. Wang Z, Li X, Feng Y, and Yang X participated in the data analysis. Qu W, Yang B, and Lin Q conceived and guided the program. All authors contributed to the general discussion.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information Experimental details and supporting data are available in the online version of the paper.
Lin Guan is currently a PhD candidate at the State Key Laboratory of Supramolecules, Jilin University. Her current research focuses on the design of adhesive hydrogel materials as well as their applications for diabetic wound dressing.
Wenrui Qu is a deputy chief physician at the Department of Hand Surgery, the Second Hospital of Jilin University, a doctor of medicine, and a postgraduate tutor. He received his PhD degree from Jilin University and worked as a visiting scholar at the School of Medicine, Indiana University-Purdue University Indianapolis, from 2013 to 2016. His research focuses on mechanisms of neuroprotection, nerve regeneration and diabetic neuropathy and unique repair strategies to promote healing of diabetic wound.
This article is excerpted from the SCIENCE CHINA Materials by Wound World.


