Charcot neuro-osteoarthropathy (CN) appears to be an uncommon diabetic foot complication which may in part be due to delay/failure to diagnose or misdiagnosis and going unrecognised, resulting in gross foot deformity. Frequently, the deformity occurs in the midfoot, producing the well described “rocker bottom foot”, however, deformity is not limited to the midfoot.
Characteristically, CN presents in those with neuropathy as a pink, warm/hot and swollen foot, and lower leg. Paradoxically, pain may be described but is not a common feature; however, when present, it is frequently described as a dull ache in the lower leg. This latter symptom in conjunction with a swollen warm limb sometimes leads to the misdiagnosis of deep vein thrombosis (DVT). Additionally, deformity may sometimes be present, generally in the mid-foot which may be subtle. An increased skin temperature of 2°C at local site(s) in the affected foot compared with identical contralateral locations has been used as a diagnostic threshold for active CN (Jones et 2023). In our experience, however, is it more likely to observe temperature differences of 4–6°C.
X-ray examination in the early stages will show generalised demineralisation and typically fractures of the metatarsals or tarsal bones. One early radiological sign that can be seen before fractures or bony destruction is a gap at the base of the first and second metatarsals, signifying rupture of the Lisfranc ligament.
The true incidence and prevalence of CN in diabetes mellitus is unknown. There are UK reported prevalence rates of 0.04% pooled data from seven foot-care specialist centres in England (Metcalf et al, 2018), 0.3% at a regional referral centre in Ireland (O’Loughlin et al, 2017) and 0.53% from a national registry study in Denmark (Svendsen et al, 2021). Thus, it appears that although diabetic peripheral polyneuropathy is common, CN appears to be comparatively rare. The exact pathogenesis of CN is still not fully understood; however, the presence of an unusually good blood supply and neuropathy are accepted to be prerequisites (Rajbhandari et al, 2002).
A novel theory (Jeffcoate et al, 2005) has clearly directed that CN is to be viewed as an inflammatory process affecting bone and soft tissues in persons with peripheral polyneuropathy. Subsequent studies have supported this theory of CN being an inflammatory condition (Mabilleau et al, 2008; Uccioli et al, 2010; Petrova et al, 2014; 2015; Jeffcoate and Game, 2022). This again supports the necessity of good arterial supply to mount this extraordinary and prolonged inflammatory response to unperceived injury.
Sadly, due to the abnormal inflammatory nature of this condition, delayed diagnosis and suboptimal management, the acute phase of CN can be protracted. In these situations, this may result in gross foot deformity, which in some instances can warrant lower-limb amputation. That aside, the resultant deformed and biomechanically dysfunctional foot is prone the abnormal plantar forces, resulting in difficult to heal foot ulcers and footwear fitting problems. There has been a reported 6 to 12 times increased risk of major amputation in those ulcerated CN compared to those without ulceration (Wukich et al, 2017; Sohn et al, 2010). Furthermore, pooled data from studies estimate a mean 5-year mortality of 29% in those suffering with CN (Armstrong et al, 2020).
Anecdotally, it is accepted that CN does not occur in the presence of peripheral vascular disease and, in fact, a number of studies have demonstrated a greater hyperaemic microvascular blood flow in patients with CN compared with those with neuropathy alone (Shapiro et al, 1998; Veves et al, 1998; Baker et al, 2007).
We report a unique case report of CN, following arterial bypass surgery in both limbs, which supports the concept that a good blood supply is necessary in the development of CN.
Case report
A 61-year-old female presented with a 1.5cm² neuroischaemic ulcer of the plantar surface of the right first metatarsal head associated with intermittent claudication in that the same leg. She had a 22-year history of type 1 diabetes complicated by peripheral polyneuropathy, retinopathy, nephropathy, peripheral arterial disease (PAD), hypertension and ischaemic heart disease.
Neuropathy was confirmed by an inability to detect vibration and 10g pressure. Clinically PAD was evident by a weakly palpable right posterior tibial pulse, absent dorsalis pedis and intermittent calf claudication. The ankle brachial pressure index (ABPI) measured at the posterior tibial artery was 0.58 on the right and 0.9 on the left, confirming right-sided arterial disease.
A femoral angiogram demonstrated a significant and moderate stenosis in the right superficial femoral artery (SFA) and the distal popliteal artery, respectively. The SFA was successfully treated with a transluminal angioplasty with subsequent ulcer healing. Unfortunately, 6 months later, the claudication and ulcer re-occurred. Repeat femoral angiogram revealed a moderate stenosis in the right external iliac artery, re-stenosis of the SFA and a complete occlusion of the distal popliteal artery. The SFA was successfully treated again by transluminal angioplasty; however, following a multidisciplinary vascular radiology team meeting, this was considered insufficient and a right femoropopliteal bypass was subsequently performed. The patient recovered uneventfully and, with offloading and regular wound-care, her ulcer healed within 6 weeks.
Two years later, she developed a hot, painful, right foot. There was no history of trauma but her foot was swollen and 4 ºC hotter than the contralateral limb, and had a subtle deformity of medial cuneiform-first metatarsal joint. A Doppler ultrasound examination of the right leg confirmed that the graft arteries were still patent and the ABPI was 1.03. A foot X-ray showed a fracture of the medial cuneiform with widening of the bases of the first and second metatarsals characteristic of Lisfranc ligament rupture (Figure 1).
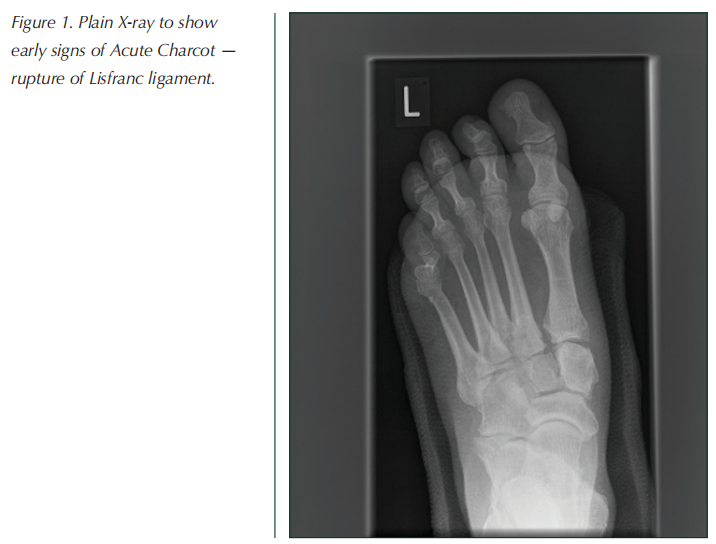
A diagnosis of CN was made and the limb was immobilised for approximately 8 months until the CN became quiescent; despite this, her right mid-foot collapsed causing the typical deformity of CN. She has had no further problems with this foot being managed by regular podiatry and bespoke footwear.
Later that year, she developed a new ulcer on the heel of her contralateral left foot. Her pedal pulses were palpable but her ABPI on that side had deteriorated within 12 months from 0.87 to 0.5. A femoral angiogram showed multiple stenoses in the left SFA and a long occlusion of the popliteal artery. These were successfully treated with a fem-distal bypass. Despite the ulcer healing well, 8 weeks later, she developed pain and swelling in the left midfoot which was found to be 3ºC hotter than the contralateral limb with no obvious deformity.
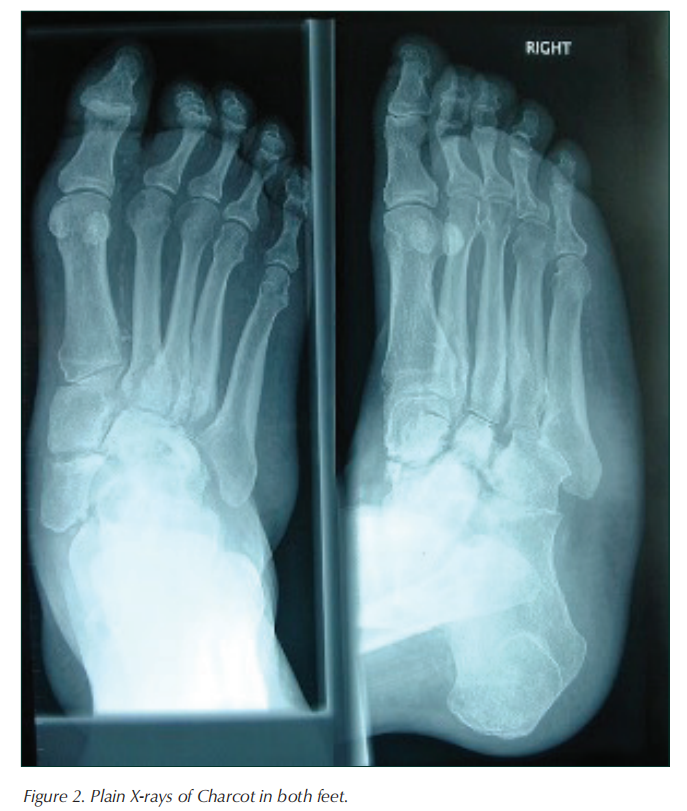
Initially, infection was considered, but microbiological cultures of biopsied tissue were negative. The foot X-ray showed fractures at the base of the second and third metatarsals with no features consistent with infection anywhere in her foot. The affected limb was immediately placed into a below-knee cast with weekly changes for wound -care to her heel ulcer that healed uneventfully. Serial X-rays showed typical changes in keeping with CN, thus confirming our diagnosis of CN. Despite immobilisation within a non-removable cast, she developed characteristic mid-foot deformity. She remained in a cast for 6 months while waiting for her skin temperature to reduce to that of her contralateral limb. She was provided with made-to-measure footwear and orthoses, and had no further foot ulcers or CN relapse.
Conclusion
This case clearly illustrates the importance of a good blood supply in the development of CN and, to our knowledge, is the first reported case of bilateral CN following revascularisation to both legs. It also shows that regular follow-up following revascularisation is essential, not just to review flow patency, but in those with peripheral neuropathy.
References
1. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA (2020) Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res 13(1):16
2. Baker N, Green A, Krishnan S, Rayman G (2007) Microvascular and C-fibre function in diabetic Charcot neuroarthropathy and diabetic peripheral neuropathy. Diabetes Care 30(12): 3077–9
3. Jeffcoate W, Game F (2022) The Charcot Foot Reflects a Response to Injury That Is Critically Distorted by Preexisting Nerve Damage: An Imperfect Storm. Diabetes Care 45(7): 1691–7
4. Jeffcoate WJ, Game F, Cavanagh PR (2005) The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet 366(9502): 2058–61
5. Jones PJ, Davies MJ, Webb D et al (2023) Contralateral foot temperature monitoring during Charcot immobilisation: A systematic review. Diabetes Metab Res Rev e3619
6. Mabilleau G, Petrova NL, Edmonds ME, Sabokbar A (2008) Increased osteoclastic activity in acute Charcot’s osteoarthropathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia 51(6): 1035–40
7. Metcalf L, Musgrove M, Bentley J et al (2018) Prevalence of active Charcot disease in the East Midlands of England. Diabet Med 35(10): 1371–4.
8. O’Loughlin A, Kellegher E, McCusker C, Canavan R (2017) Diabetic charcot neuroarthropathy: prevalence, demographics and outcome in a regional referral centre. Ir J Med Sci 186(1): 151–6
9. Petrova NL, Petrov PK, Edmonds ME, Shanahan CM (2015) Inhibition of TNF-alpha Reverses the Pathological Resorption Pit Profile of Osteoclasts from Patients with Acute Charcot. Osteoarthropathy. J Diabetes Res 2015: 917945
10. Petrova NL, Petrov PK, Edmonds ME, Shanahan CM (2014) Novel use of a Dektak 150 surface profiler unmasks differences in resorption pit profiles between control and Charcot patient osteoclasts. Calcif Tissue Int 94(4): 403–11
11. Rajbhandari SM, Jenkins RC, Davies C, Tesfaye S (2002) Charcot neuroarthropathy in diabetes mellitus. Diabetologia 45(8): 1085–96
12. Shapiro SA, Stansberry KB, Hill MA et al (1998) Normal blood flow response and vasomotion in the diabetic Charcot foot. J Diabetes Complications 12(3): 147–53
13. Sohn MW, Stuck RM, Pinzur M et al (2010) Lower-extremity amputation risk after charcot arthropathy and diabetic foot ulcer. Diabetes Care 33(1): 98–100
14. Svendsen OL, Rabe OC, Winther-Jensen M, Allin KH (2021) How Common Is the Rare Charcot Foot in Patients With Diabetes? Diabetes Care 44(4): e62-e
15. Uccioli L, Sinistro A, Almerighi C et al (2010) Proinflammatory modulation of the surface and cytokine phenotype of monocytes in patients with acute Charcot foot. Diabetes Care 33(2): 350–5
16. Veves A, Akbari CM, Primavera J et al (1998) Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 47(3): 457–63
17. Wukich DK, Sadoskas D, Vaudreuil NJ, Fourman M (2017) Comparison of Diabetic Charcot Patients With and Without Foot Wounds. Foot Ankle Int 38(2): 140–8
This article is excerpted from the 《The Diabetic Foot Journal Vol 26 No 1 2023》by Wound World.


