1. Heel ulcers are more common than you may realise.
Some of the largest samples of pressure ulcer data are from many years of work by VanGilder (2021), including a voluntary point prevalence study (International Pressure Ulcer Prevalence [IPUP] Survey) completed in US acute care hospitals, which yielding rich data for study. An in-depth analysis of data between 2018 and 2019 of 2,703 unique facilities (1,179,108 patients) revealed that the most common heel pressure ulcer was deep tissue pressure injury. When all stages and sites were examined in hospital patients, the heels were the most common at 30% of pressure ulcers.
A secondary analysis, which examined only critical care patients (n=41,866), identified that deep tissue pressure injury was the most common type of pressure ulcer on the heel, at 12.8% (Cox et al, 2022).
2. The anatomy of the heel and its native blood flow create a unique risk for injury.
The calcaneus is prominent, that is, when the leg is lying on a bed, the calf flattens out and the calcaneus protrudes. There is also no direct blood flow to the heel. The majority of the blood supply to the heel is provided by the posterior tibial artery, and only to a small extent by the posterior branch of peroneal artery. These arteries are the first ones involved with peripheral arterial disease (PAD).
3. The risk for heel pressure ulcer development is not always obvious.
In a large database study, the average Braden Scale for Predicting Pressure Sore Risk score for patients with heel ulcers was found to be 17. (The Braden scale rates risk in six areas: level of consciousness and sensation, exposure to moisture, ability to move in bed, ability to move out of bed, nutrition and exposure to friction and shear. Scores range from 6–23, with the higher number indicating less risk.) A score of 17 would generally indicate little risk. The Waterlow scale has a specific subscale that focuses on duration of surgery, but no data could be found on its use and heel ulcers. So why is the risk not identifiable? Immobility is the leading risk factor for pressure ulcers in general, and that is true for the heel also. Single leg immobility from having knee replacement surgery puts the heel at risk. So, in those patients, other risk factors such as incontinence and malnutrition do not always play a major role. In addition, the neuropathy of diabetes is not always factored into the risk and peripheral arterial disease is not always recognised. Leg immobility, neuropathy and poor arterial flow are the big three variables.
4. Peripheral arterial disease increases risk for heel pressure ulcers
When pressure is applied to any tissue, the tissue can become ischaemic. Only when the pressure is removed, can arterial blood flow into the tissues and remove oxygen debt and cellular waste. When arteriosclerosis is present, the stiff blood vessels cannot dilate effectively, and the oxygen debt will remain in effect longer. Arterial blood flow will enter the tissues eventually, except in patients with critical limb ischaemia. In a matched case-control study, 15 patients identified as having a grade 2, 3 or 4 pressure ulcer of the heel and were compared with 15 matched controls without pressure ulcers of the heel. Participants were identified as having PAD where the ankle brachial pressure index (ABPI) was <0.9 or >1.3. Patients presenting with pressure ulcers of the heel were significantly more likely to simultaneously have previously undiagnosed PAD compared with age, gender, and ethnicity matched controls without pressure ulcers of the heel (odds ratio: 11; Twilley and Jones, 2016). The time required to recover oxygen debt in patients’ heels is unknown.
5. Diabetic peripheral neuropathy is a significant risk factor for heel pressure ulcers
Several aspects of diabetes increase risk for pressure ulcer on the heels; diabetic peripheral neuropathy (DPN) being a major aspect. Hyperglycaemia and dyslipidaemia are the primary causes of DPN in patients with type 1 and 2 diabetes, respectively. The prevalence of diabetic peripheral neuropathy (DPN) ranges from 6% to 51% depending on the population. It has been estimated that half of all children with diabetes with a duration of 5 years or longer already have DPN and nearly 25% of paediatric patients with newly diagnosed diabetes have abnormal findings on nerve conduction studies. The prevalence of DPN is somewhat higher in patients with type 2 diabetes when compared with type 1 diabetes. DPN has been shown to be present in 42% and 39% of adults with type 2 diabetes, respectively, at baseline measurement (Carmicheal et al, 2021).
Patients with neuropathy of any cause, such as paralysis, multiple sclerosis or diabetes have significantly reduced or absent sensation in the feet. They are unaware of pressure on the heels and, therefore, do not move their legs. Due to the rapid onset of DPN, any patient with diabetes should be classified as at risk for heel pressure injury.
6. Diabetes also alters heel skin blood flow, further increasing risk for pressure ulcers.
Diabetes accelerates atherosclerosis, which impairs blood flow. A study of heel skin microcirculation quantitatively assessed return of blood flow directly and after 3 and 6 minutes following relief of pressure using laser Doppler flowmetry and tissue spectrophotometry. Normal reactive hyperaemia occurred immediately after relief of pressure, as measured by blood flow increased in the superficial skin layers (2mm below the surface of the skin) in both groups. However, in deep skin layers (8mm below the surface of the skin), blood flow increased in patients with diabetes mellitus and decreased in healthy patients. Oxygen saturation (SO2 ) was higher in healthy subjects directly after pressure relief. In contrast, prolonged hyperaemia in deep skin layers in patients with diabetes indicates that there was increased tissue vulnerability (Held et al, 2019).
7. The skin of the foot and heel is abnormally stiff in patients with diabetes, further increasing risk of skin ulceration.
Heel skin in patients with diabetes become thicker and less tolerant of pressure. As discussed above, impaired sensation and blood flow are significant risk factors in patients with diabetes. However, Gefen (2010) described that soft tissue in the heel becomes thick and stiff due to interlinking collagen fibres. These stiff tissues cannot offload pressure, further decreasing a tolerance to pressure and shear forces.
8. Pressure ulcers on the heel can heal.
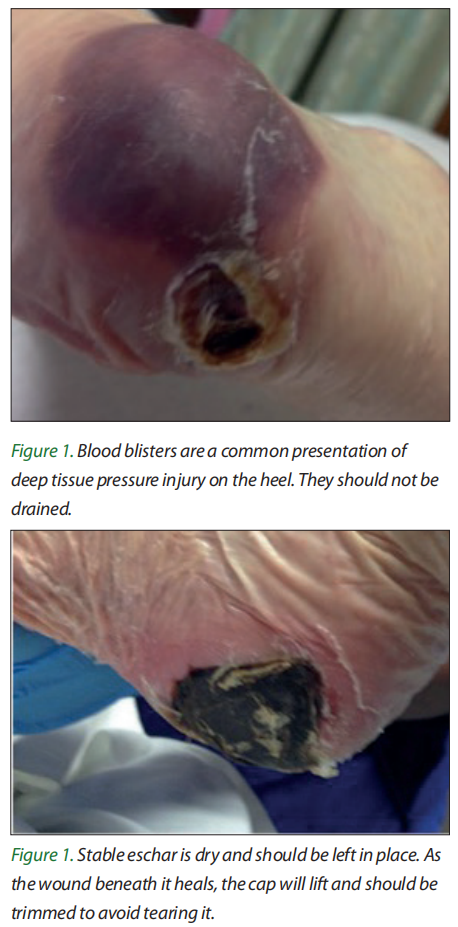
Many pressure ulcers on the heel begin as deep tissue pressure injury, sometimes seen as intact purple tissue or later as blood blisters [Figure 1]. If the blood blister is resorbed and not drained, it creates a hard tissue cap, called a stable eschar [Figure 2]. This tissue should not be debrided unless it becomes infected. The patient with a heel ulcer as commonly described as above, is an individual with diabetes with arterial disease and an immobile leg. Nothing intrinsic is present to support healing! So, leave the eschar cap in place and watch and wait, if the cap opens or infection is present, it should be removed. If any healing occurs, the edges of the cap will lift and can be trimmed. Tell the patient this process will take over 6 months and not to soak or soften that eschar cap.
A study designed to examine the interventions that aid healing in ischaemic heel ulcers was completed by Treiman and colleagues (2000). The authors examined 91 patients that had heel wounds which had not healed in up to 12 months. Ankle brachial indices (ABI) were low, and the mean preoperative ABI was 0.51. Thirty-one percent of the wounds were infected. Other factors common to the non-healing group included diabetes in 70%, impaired renal function in 55% (Cr > 1.5), with 24% undergoing dialysis, and 64% smoked cigarettes. Nearly all patients had some type of revascularisation of the posterior tibial artery. Restoring blood supply, of course, increased ABI and healing within 6 months. All wounds with occluded grafts failed to heal and 11% of patients required amputation.
Diabetes also delays healing. Faglia and colleagues (2013) reported that their patients with a heel ulcer have a higher risk of major amputation compared with patients with lesions of the midfoot and forefoot.
9. Expand your assessment of the heel.
When assessing the skin of the heel, be sure you look for a nonblanchable redness or purple/maroon discolouration. Sometimes you need to use a mirror to see the skin of the heel in bedridden patients. At times, the patient can turn in bed or stand, so the heel can be inspected. In addition, when factoring in arterial disease, palpate the temperature of the foot, determine if the dorsalis pedis and posterior pulses are palpable. If not palpable use a Doppler to find them. Assess the quality of the skin and hair (is it shiny? Are other ulcers present? is hair present?). Determining ABI can be very helpful to determine the level of vascular flow. When assessing for neuropathy, ask the patient about sensation in the feet and heels. Monofilament testing can also determine if the patient has or does not have protective sensation.
10. Prevention of heel ulcers is crucial.
Due to increasing numbers of patients with diabetes and the effect of that disease on neuropathy and blood flow, it is imperative that every patient with diabetes who is at bedrest has heel protection. Further, a number of patients in critical care are on vasopressors, which exacerbates leg ischemia as vasopressors shunt blood to vital organs.
The choice of products to use on the heel varies widely. Pillows can be used for patients who do not move their legs; however, pillows rapidly collapse and leave the heel on the pillowcase on the bed. Naturally, if the patient moves their legs, the pillows will be on the floor. Heel off-loading devices (HOLD) are very effective if the device frees the heel from pressure. When considering these devices, examine the heel portion; is the heel floating or wrapped in the boot? Putting a stocking on the heel does not offload the heel. Sheepskin heel “booties” provide no heel protection. Some foam heel dressings can reduce pressure and shear, but the preferred method is to offload the heel from the bed. Use caution when ambulating patients wearing these devices; they are often not meant to be worn when walking.
There are only a handful of patients who cannot have their heels floating, including patients in external fixation devices for lower leg fractures, patients with end stage arterial disease, who develop ischaemic pain when the leg is elevated, and patients in cardiogenic shock, who cannot have their legs elevated to avoid intense venous return. These patients are usually well managed with foam dressings on the heels.
Conclusion
Pressure ulcers on the heels are unfortunately common in the group of patients with poor healing potential. Fortunately, they are some of the easiest pressure ulcers to prevent. We must play our part to teach our patients and staff how to reduce risk by floating the heel. Risk assessments won’t help us — it must instead be our commitment to our patients.
References
1. Carmichael J, Hassan Fadavi H, Ishibashi F et al (2021) Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol (Lausanne) 12: 671257. https://doi.org/10.3389/ fendo.2021.671257
2. Cox J, Edsberg LE, Koloms K, VanGilder CA (2022) Pressure injuries in critical care patients in us hospitals: results of the international pressure ulcer prevalence survey. J Wound Ostomy Continence Nurs 49(1): 21–28 https:// doi.org/10.1097/won.0000000000000834
3. Faglia E, Clerici G, Caminiti M (2013) Heel ulcer and blood flow: the importance of the angiosome concept. Int J Low Extrem Wounds 12(3): 226–30
4. Gefen A (2010) The biomechanics of heel ulcers. J Tissue Viability 9(4): 124-31
5. Held M, Bender D, Krauß S et al (2019) Quantitative analysis of heel skin microcirculation using laser Doppler flowmetry and tissue spectrophotometry. Adv Skin Wound Care 32(2): 88–92
6. Treiman GS, Oderich GS, Ashrafi A, Schneider PA (2000) Management of ischemic heel ulceration and gangrene: An evaluation of factors associated with successful healing. J Vasc Surg 31(6): 1110–8
7. Twilley H, Jones S (2016) Heel ulcers - Pressure ulcers or symptoms of peripheral arterial disease? An exploratory matched case control study. J Tissue Viability 25(2): 150–6
8. VanGilder CA, Cox J, Edsberg LE, Koloms K (2021) Pressure Injury Prevalence in Acute Care Hospitals With Unit-Specific Analysis: Results From the International Pressure Ulcer Prevalence (IPUP) Survey Database. J Wound Ostomy Continence Nurs 48(6): 492–503
This article is excerpted from the Wounds International 2023 | Vol 14 Issue 2 | by Wound World.


