Introduction
Diabetes is a well-known risk factor for major cardiovascular disease (CVD) and is deemed to increase at least twice the risk of atherosclerotic disease compared with non-diabetic patients [1–4].
Aspirin is commonly used in the treatment and prevention of CVD, and the effectiveness of aspirin for the secondary prevention of CVD is well established in people with or without diabetes. In contrast, the role of aspirin in primary prevention is still controversial, and conceptually an overall benefit can be achieved if the baseline risk is high enough to outweigh the risks of aspirin side effects. Diabetic patients were deemed to fulfill these criteria but dedicated randomized controlled trials failed to show a clear net benefit of aspirin for the primary prevention of CVD in people with diabetes [5].
Nevertheless, guidelines still suggest that aspirin might be considered for the primary prevention in selected patients at higher CVD risk but not at increased bleeding [6].
In order to further explore the risk of CVD risk among diabetic patients and the putative benefit hypothesis of aspirin in this population at primary prevention we conducted a retrospective analysis of data from VITAL (Vitamin D and Omega-3 Trial) cohort, majorly composed by patients with less than 70 years [7].
Subjects, materials, and methods
This was a retrospective evaluation of the VITAL trial using data provided by the Project Data Sphere (https://doi.org/10. 34949/n4c7-zm25). Detailed methodology and main results of the trial has been published elsewhere [8]. In summary, 25 828 adults (men age≥50 and women age≥55 years) were followed for a median of 5.3 years (range, 3.8–6.1 years), since July 2010. VITAL was a 2×2 factorial double-dummy randomized controlled trial (RCT) evaluating Vitamin D and/or omega-3 fatty acids vs placebo for primary prevention of cancer and cardiovascular disease. The dataset used is de-identified and public according to request to PDS. This project complies with the principles of the Declaration of Helsinki as revised in 2008.
Participants
The VITAL trial was performed in the United States in patients at primary prevention. Therefore, participants were required to have no history of cancer (except non-melanoma skin cancer), myocardial infarction, stroke, transient ischemic attack (TIA), angina pectoris, or coronary revascularization. In addition, participants were required to limit consumption of supplemental vitamin D, to limit consumption of supplemental calcium, and to forego the use of fish oil supplements during the run-in and randomized treatment periods. Patients with renal failure or dialysis, hypercalcemia, hypo- or hyperparathyroidism, severe liver disease (cirrhosis), or sarcoidosis or other granulomatous diseases, such as active chronic tuberculosis or Wegener's granulomatosis, were excluded [8].
For our retrospective analysis participants were split according to the diagnosis of diabetes at admission and aspirin use.
Outcomes
The primary method of follow-up was by mailed questionnaires and reviewing medical records to confirm study endpoints. Participants received follow-up questionnaires at 6 months and 1 year after randomization and annually thereafter. An Endpoints Committee of physicians who were blinded to the randomized treatment assignment reviewed the medical records to confirm or refute the case by applying a defined protocol [8].
The primary cardiovascular outcome of the VITAL study was major cardiovascular adverse events (MACE), a composite endpoint of myocardial infarction, stroke, and death from cardiovascular causes. The definition of Myocardial infarction (MI) was that of Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the Redefinition of Myocardial Infarction criteria. Stroke was diagnosed and categorized according to Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria. Cardiovascular deaths were confirmed by convincing evidence of a CVD event from all available sources, including death certificates, hospital records, autopsy reports, and, for deaths outside the hospital, observer accounts. Total coronary heart disease (CHD) was a composite of myocardial infarction, coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), and death from coronary heart disease. Expanded MACE was a composite of MACE and coronary revascularization (coronary artery bypass grafting or percutaneous coronary intervention) [8, 9].
Statistical methods
Participants of the cohort were split according with the baseline diabetes status and in a second phase the diabetic participants were split according to aspirin usage. Descriptive analysis was performed, mean and standard deviation were presented for continuous variables and absolute and relative frequencies for categorical variables. Statistical comparisons were performed using t-test for independent variables to test for continuous values and Chi-square test for categorical variables. Cox regression models were applied to assess the risk estimates for univariate models and multivariate models. The tied events were handled through the Breslow method, and the Schoenfeld residuals were used to test the assumption of proportional hazards method. Hazard Ratio (HR) and 95%CI were estimated. A significance level of 5% was assumed for all statistical calculations. Analyses were performed using the STATA statistical software program (STATA version 17.0; StataCorp, College Station, TX).
Results
Diabetes risk for cardiovascular events in the VITAL cohort
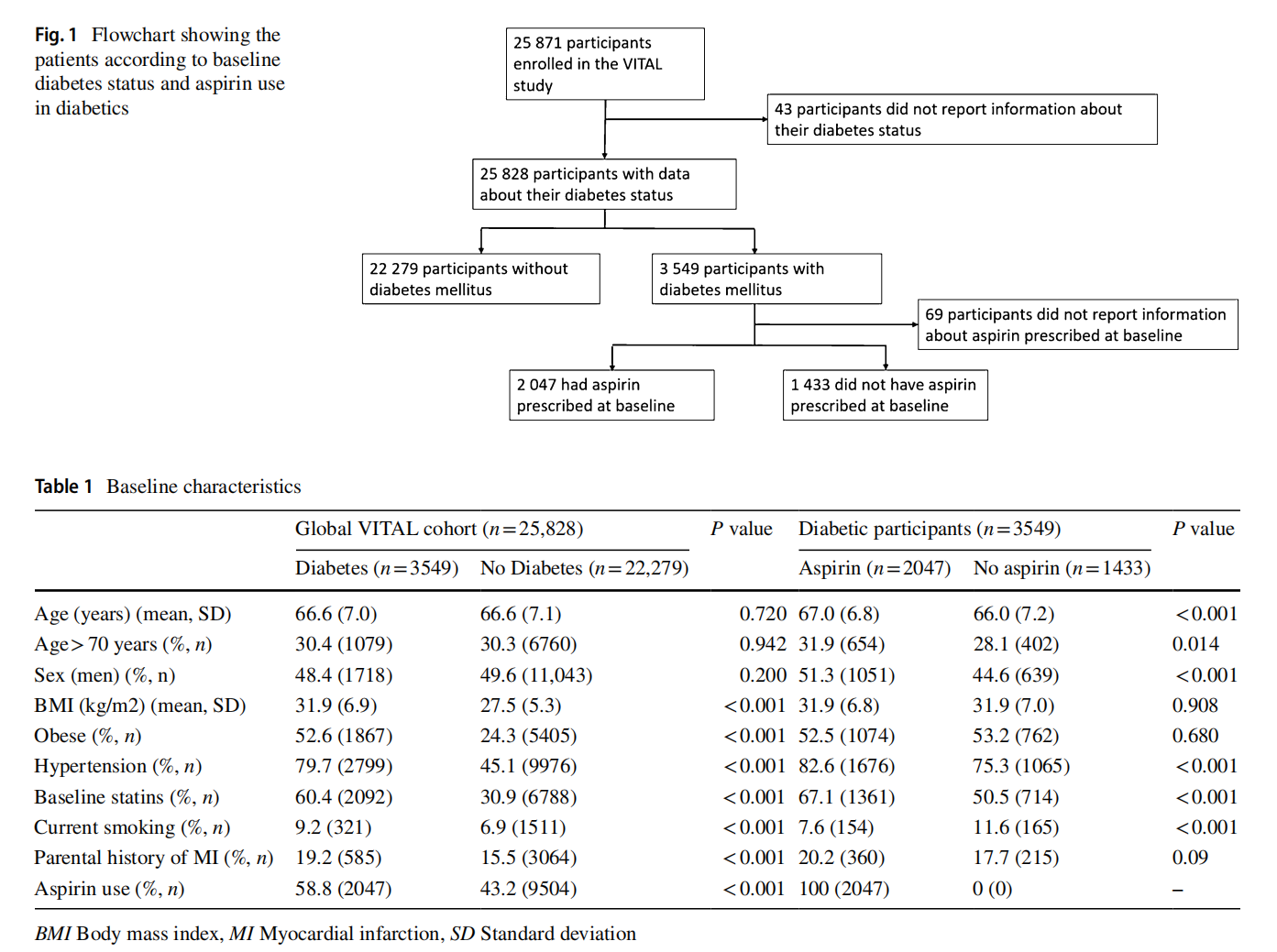
Among 25,828 participants with information about the baseline diabetes status, 13.7% (n=3549) were diabetic (Fig. 1). The mean age of the participants was 66.6 years and there were no significant differences regarding age in diabetics and non-diabetic participants, nor in the proportion of male/ female participants. Diabetic patients had a higher prevalence of other cardiovascular risk factors, such as obesity, hypertension, dyslipidemia, current smoking, and parental history of MI (Table 1). The frequency of aspirin use was higher among patients with diabetes compared with nondiabetics (58.8% vs. 43.2%) in this primary prevention population (Table 1).
After adjusting for potential confounder, people with diabetes had increased risk of mortality (HR 1.61, 95%CI 1.33–1.94) and higher risk of cardiovascular outcomes, such as MACE (HR 1.36 95%CI 1.11–1.68), expanded MACE (HR 1.37, 95%CI 1.15–1.63), MI (HR 1.47, 95%CI 1.08–2.02), coronary heart disease (HR 1.39, 95%CI 1.12–1.73), and stroke (HR 1.54, 95%CI 1.09–2.18), when compared to non-diabetic population (Fig. 2). Figure 3 shows the cumulative hazard plot of MACE comparing diabetic and non-diabetic individuals. Figure 2 shows in the left side of the plot the unadjusted and adjusted estimates associated with diabetes compared to non-diabetic participants. Supplementary Table 1 shows the number of events and the incidence rate among the groups.


Association of baseline aspirin use in diabetic patients with cardiovascular events
Among participants with diabetes, aspirin users were older (67 vs 66 years old) and more frequently men (51 vs 45%, p<0.001). There were no differences regarding obesity between the groups. At baseline 82.6% of aspirin users had hypertension, significantly higher compared to non-users (vs. 75.3%, p<0.001). The proportion of statins users and current smokers was also higher in diabetics treated with aspirin (Table 1).
After adjusting for age, sex, body mass index, smoking status, parental history of myocardial infarction, hypertension, and statins use, aspirin was not associated with decreased risk of cardiovascular events (MACE: HR 0.92, 95%CI 0.64–1.33) nor all-cause mortality (HR 0.81, 95%CI 0.59–1.11) (Fig. 2). Figure 2 shows in the right side of the plot the unadjusted and adjusted estimates associated with aspirin use diabetic patients. Supplementary Table 1 shows the number of events and the incidence rate among the groups.
Discussion
In this post hoc retrospective analysis of the VITAL study, we confirmed that diabetes had a significant impact in the mortality and cardiovascular outcomes among individuals at primary prevention and that aspirin use in diabetic patients in this setting was not associated with risk reduction of mortality or cardiovascular events.
It is important to stress that patients treated with aspirin had high frequency of cardiovascular risk factors, such as older age, male gender, hypertension, and smoking habits. Data from diabetes vs no diabetes are concordant with literature and support the increased risk of cardiovascular events associated with diabetes [1], and at least partially explain the willingness to prescribe aspirin in these patients [10]. However, the magnitude of risk increase with diabetes was not high as previously reported [4], which can be related to the standard practices for care of cardiovascular risk factors. And despite the adjustments of estimates for different potential cofounder the putative expected benefit of aspirin in diabetic patients was not documented in this cohort.
Previous systematic review of RCTs, including more than 27,000 diabetic patients, suggested that aspirin use among people with diabetes increased risk of major bleeding and major gastrointestinal bleeding with a modest 8% risk reduction of MACE and no mortality benefit [11]. Additionally, the small benefit of aspirin mostly relied on older and small clinical trials, while recent trials, such as ASCEND, showed that the risk–benefit of aspirin in contemporary population might be weak [12]. Oppositely to the data from RCT, in observational studies the groups might not balanced in terms of cardiovascular risk factors and comparison are at higher risk of bias also due to possible indications bias related to aspirin prescription (i.e., aspirin is prescribed preferentially in individuals at higher cardiovascular risk). In our analyses we attempted to adjust for these potential sources of bias, but we acknowledge that residual bias can affect the estimation of the results.
After many years promoting aspirin benefits among patients, deprescribing aspirin in primary prevention in patients with low bleeding risk could be challenging to physicians. Although data regarding cardiovascular events after deprescribing aspirin in low/moderate CVD risk patients is lacking, previous Swedish study stated that discontinuing primary prevention aspirin in general population was associated with a 28% higher rate of cardiovascular events than continuing on aspirin, which result in an absolute risk increase of 6.9 per 1000 person-years at risk or an additional cardiovascular event per year in 1 of every 146 patients who discontinued aspirin [13]. However, no data about bleeding complications risk prevented by deprescription were supplied.
Our retrospective analysis of the VITAL cohort has some limitation that should be acknowledged. First it is important to notice that VITAL participants were health-conscious group of people, keen to participate in a clinical trial with the intention to reduce their CVD or cancer risk. [14] So, data could not be generalized to less health-conscious people and with possibly very high CVD risk. Another limitation of our study is the retrospective nature of our analysis and the possible residual bias despite the adjustments made in the analyses. The lack of comprehensive data regarding overall major bleeding risk, in particular gastrointestinal bleeding, could also be a pitfall.
Conclusions
This retrospective analysis of the VITAL study showed that diabetes significantly increases the risk of cardiovascular events in a contemporary cohort of patients at primary prevention. No association was found between aspirin and cardiovascular benefits of aspirin in these diabetic patients at primary prevention.
Supplementary Information
The online version contains supplementary material available at https:// doi.org/10.1007/s40618-022-02001-3.
Acknowledgements
This publication is based on research using information obtained from www.proje org, which is maintained by Project Data Sphere. Neither Project Data Sphere nor the owners of any information from the web site have contributed to, approved, or are in any way responsible for the contents of this publication.
Author contributions
DC was involved in substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. MA was involved in substantial contributions to analysis and interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published. JJF and FJP were involved in substantial contributions to interpretation of data; revising the article critically for important intellectual content; and final approval of the version to be published.
Funding Open access funding provided by FCT|FCCN (b-on). None.
Data availability We used the data provided by the Project Data Sphere (https://doi.org/10.34949/n4c7-zm25). Detailed methodology and main results of the trial have been published elsewhere. The dataset used is de-identified and public according to request to PDS.
Declarations
Conflict of interests DC has participated in educational meetings and/or attended a conferences or symposia (including travel, accommodation and/or hospitality) with Bial, Bristol-Myers Squibb, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck Serono, Ferrer, Pfzer, Novartis and Roche. JJF is a consultant for Ipsen, GlaxoSmithKline, Novartis, Teva, Lundbeck, Solvay, Abbott, BIAL, Merck Serono, and Merz and received grants from GlaxoSmithKline, Grunenthal, Teva, and Fundação MSD. FJP had consultant and speaker fees with Astra Zeneca, Bayer, BMS, Boehringer Ingelheim and Daiichi Sankyo. The remaining authors have nothing to declare.
Research involving human participants and/or animals and informed consent This was a retrospective evaluation of the VITAL trial using data provided by the Project Data Sphere (https://doi.org/10.34949/ n4c7-zm25). Detailed methodology and main results of the trial have been published elsewhere. The dataset used is de-identified and public according to request to PDS. This project complies with the principles of the Declaration of Helsinki as revised in 2008.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reference
1. Yusuf S, Hawken S, Ôunpuu S et al (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364(9438):937–952. https://doi.org/10.1016/S0140-6736(04) 17018-9
2. Huxley R, Barzi F, Woodward M (2006) Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332(7533):73–78. https://doi.org/10.1136/bmj.38678.389583.7C
3. O’Donnell MJ, Chin SL, Rangarajan S et al (2016) Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 388(10046):761–775. https://doi.org/10.1016/ S0140-6736(16)30506-2
4. Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease the framingham study. J American Med Associat. 241(19):2035–2038
5. ASCEND Study Collaborative Group, Bowman L, Mafham M et al (2018) Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 379(16):1529–1539
6. Visseren FLJ, Mach F, Smulders YM et al (2021) 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 42(34):3227–3337. https://doi.org/10.1093/eurhe artj/ehab484
7. White E, Patterson RE, Kristal AR et al (2004) VITamins and lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol 159(1):83–93. https://doi.org/10. 1093/AJE/KWH010
8. Manson JAE, Bassuk SS, Lee IM et al (2012) The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 33(1):159–171. https:// doi.org/10.1016/J.CCT.2011.09.009
9. Manson JE, Cook NR, Lee IM et al (2019) Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 380(1):33–44. https://doi.org/10.1056/NEJMOA1809 944
10. Chen Y, Yin C, Li Q et al (2021) Misuse of Aspirin and Associated Factors for the Primary Prevention of Cardiovascular Disease. Frontier Cardiovas Med. https://doi.org/10.3389/fcvm.2021. 720113
11. Caldeira D, Alves M, David C, Costa J, Ferreira JJ, Pinto FJ (2020) Aspirin in the primary prevention of cardiovascular disease on diabetic patients: Systematic review and meta-analysis. Prim Care Diabetes 14(3):213–221. https://doi.org/10.1016/J.PCD. 2019.11.004
12. Khan SU, Ul Z, Asad A et al (2019) Aspirin for primary prevention of cardiovascular outcomes in diabetes mellitus : An updated systematic review and meta-analysis. Published online. https://doi. org/10.1177/2047487319825510
13. Sundström J, Hedberg J, Thuresson M, Aarskog P, Johannesen KM, Oldgren J (2017) Low-Dose Aspirin Discontinuation and Risk of Cardiovascular Events: A Swedish Nationwide. PopulBased Cohort Study Circulat 136(13):1183–1192. https://doi.org/ 10.1161/CIRCULATIONAHA.117.028321
14. Bassuk SS, Manson JAE, Lee IM et al (2016) Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials 47:235–243. https://doi.org/10. 1016/J.CCT.2015.12.022
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the Journal of Endocrinological Investigation (2023) 46:1423–1428 by Wound World.


