Introduction
Despite advances in surgical technique, adoption of procedural guidelines, increased treatment options, and better understanding of surgical wound microenvironments, surgical site infections (SSIs) remain a significant complication, and at 42.4% of all healthcare-associated infections (HAIs), have emerged as the most common HAI in the United States [1]. SSIs are the single most frequently cited reason for unplanned readmission following surgery, accounting for 19.5% of all readmission reasons across major surgical procedures and nearly 26% after colorectal surgery specifically [2].
While many efforts have been made to eliminate SSIs, up to 60% of SSIs are still considered potentially preventable with standard of care (SOC) measures that include systemic antibiotic prophylaxis [3, 4]. This may be due to issues limiting the timing and duration of target tissue penetration of SOC systemic prophylactic antibiotics. As Sheikh et al. note, “In clinical practice, plasma antibiotic concentrations are used as a surrogate marker for pharmacologic effect. However, for predicting therapeutic efficacy, the tissue concentration of antibiotic is more important than the plasma concentrations” [5]. Given that systematically measured concentrations are typically higher than those found in the subcutaneous tissues surrounding and within the wound bed [6], particularly for patients with obesity, diabetes, and peripheral vascular disease [7], low loco-regional tissue penetration, and hence duration of effect, is likely a significant and overlooked issue in SSI prophylaxis measures.
D-PLEX100 (D-PLEX) (PolyPid Ltd., Petach Tikvah, Israel) is a locally applied doxycycline formulation which pairs an innovative Polymer-Lipid Encapsulation matriX (PLEX) platform with the broad-spectrum antibiotic, doxycycline and is applied to the soft-tissue wound surfaces following fascial closure prior to skin closure (Fig. 1) for the prevention of superficial and deep SSIs. This PLEX platform contains a matrix of alternating layers of polymers and lipids, which forms a protected reservoir and enables a localized, continuous release of doxycycline for a period of 30 days [8, 9]. Here, we report a Phase 2 multi-center singleblind study evaluating the safety and efficacy of D-PLEX in addition to SOC in preventing superficial and deep surgical site infections for patients who have elective colorectal surgery.
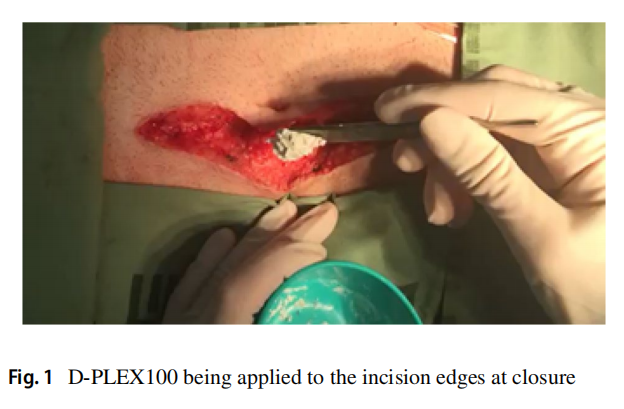
Materials and methods
Patients
The trial was conducted in 8 medical centers in Israel with each center supervised by a principal investigator. Eligible patients were adults, 18 years and older, undergoing elective colorectal surgery. Female patients of childbearing age were required to have a negative serum pregnancy test prior to the procedure. Patients planned for a laparoscopic approach were included if a 5 cm or greater incision was performed as part of the procedure and/or as a specimen extraction site. Key exclusion criteria included patients who were scheduled for emergency surgery or who had received doxycycline within the 4 weeks prior to screening. Patients undergoing concomitant surgical procedures via the same incision(s) were included pending consultation and approval of the site sponsor. Patients who had received neoadjuvant radiation to the abdominal area or systemic chemotherapy within 4 weeks of surgery were excluded. Patients with known hypersensitivity to doxycycline and/or the tetracycline family of drugs, to D-PLEX excipients, or who had allergies to more than 3 substances as determined from the screening questionnaire were also excluded.
D‑PLEX
D-PLEX is a new formulation of extended-release doxycycline, consisting of doxycycline, biodegradable polymers, and synthetic phospholipids along a beta-tricalcium phosphate backbone. Each 5 g D-PLEX vial contains 54.6 mg doxycycline. D-PLEX is supplied as a sterile powder and reconstituted with normal saline into a paste in the operating room. D-PLEX is administered as a single application, and the active material is continuously released for approximately 30 days.
Study design
The study protocol was reviewed and approved by the institutional review board at each participating site, and was implemented following the principles of Good Clinical Practice and the Declaration of Helsinki, and in accordance with International Council of Harmonization guidelines and local regulations before enrollment of participants began. All patients provided written informed consent prior to any study procedures. The study was registered at the clinicaltrials.gov: NCT03633123. The patients were randomized to receive D-PLEX administered along with SOC or the SOC control arm. The prophylactic antibiotic SOC treatment, based on the Israel Ministry of Health (IMOH) guidelines and standardized for all participating sites, included a first or second-generation cephalosporin plus metronidazole administered intravenously within 30–60 min prior to surgery. Mechanical bowel preparation (MBP) was at the discretion of the surgeon. No oral antibiotic bowel preparation (OABP) was given to either arm. For patients randomized to the treatment arm, at the time of fascia, closure D-PLEX was applied along the entire length of the surgical wound, inclusive of the fascial suture line and soft tissues of the abdominal wall, subcutaneous fat, and dermis. The D-PLEX dose was determined based on the length of the surgical incision: a 5–10 cm incision received 5 g D-PLEX, an 11–20 cm incision received 10 g D-PLEX, and an incision≥21 cm received 15 g D-PLEX.
Analysis and outcomes
Patients were randomized to either the SOC or D-PLEX plus SOC in a 1:1 ratio at day 0 via an interactive web randomization system integrated with an electronic case record form (eCRF) based on the patient’s sex, age (18–40, 41–65, and above 66), and if there was a planned ostomy creation. Patients were blinded to their study designation. Endpoints assessed included 30 day superficial and deep SSI and treatment-emergent adverse events (TEAEs). Other data collection and analyses, including pharmacokinetic data, wound microbiome, and post-operative organism colonization, were collected on varying populations and will be the discussion of a separate paper.
The incisional site was assessed at post-operative days 1, 5, 14, 30, and 60 by a blinded assessor and blinded endpoint adjudication committee (EAC). The EAC was composed of 3 physicians: 2 of whom were surgeons with expertise in colorectal surgeries and the other was an infectious diseases expert. EAC members were responsible for independently reviewing the data for each suspected SSI event and to determine whether it met the efficacy event criteria. In the event of a dispute between the blinded assessor and the committee, the committee’s adjudication prevailed. SSIs were classified following the National Healthcare Safety Network and Centers for Disease Control and Prevention Surgical Site Infection Event Reporting Manual as Superficial SSI, where the infection involved the skin and subcutaneous tissues (and not including cellulitis or stitch abscess alone) or deep SSI, when the infection involved the fascial and/or muscle layers. Organ/organ space SSIs (e.g., an intra-abdominal abscess or anastomotic leak) were assessed as TEAEs rather than SSI endpoints.
Statistical analysis
The study planned to enroll 200 patients, with 100 subjects allocated to each treatment group. This sample size was determined to provide adequate initial data for evaluating the study objectives to provide exploratory data which would form the basis of the power calculations for planned pivotal Phase 3 trials. However, exploratory sample size calculations show that a sample of 200 subjects provides 80% power to detect an 80% decrease in the SSI rate (15% versus 3%) at a two-sided α=0.10 level of significance.
Analysis consisted of summarizing the efficacy and safety data. p Values were based on a two-sided pooled Ttest of difference in treatment means or a two-sided Fisher’s exact test of difference in treatment proportions. p Values of<0.05 are considered statistically significant. All calculations were made on the combined results of all centers, and there was no selective pooling of study centers for analyses. The data analyses were conducted using SAS® Software, version 9.4 or later.
Results
From October 2018 to October 2019, 207 patients were screened and 202 proceeded for randomization to either the SOC arm (n=101) or the D-PLEX arm (n=101). Analyses reported here were performed on the per protocol population (Fig. 2). In the SOC arm, 1 patient did not proceed to surgery, 8 received preoperative oral antibiotics, and 1 was lost to follow-up. In the D-PLEX arm, 2 patients did not have D-PLEX applied at closure, 3 had dosing errors, and 8 patients were found to have other major inclusion or exclusion violations. One hundred and seventy-nine patients were evaluated in the per protocol population, 88 in the intervention arm [51 males, 37 females, median age 64.0 (range: 19–92) years] and 91 in the control arm [57 males, 34 females, median age 64.5 (range: 21–88) years].
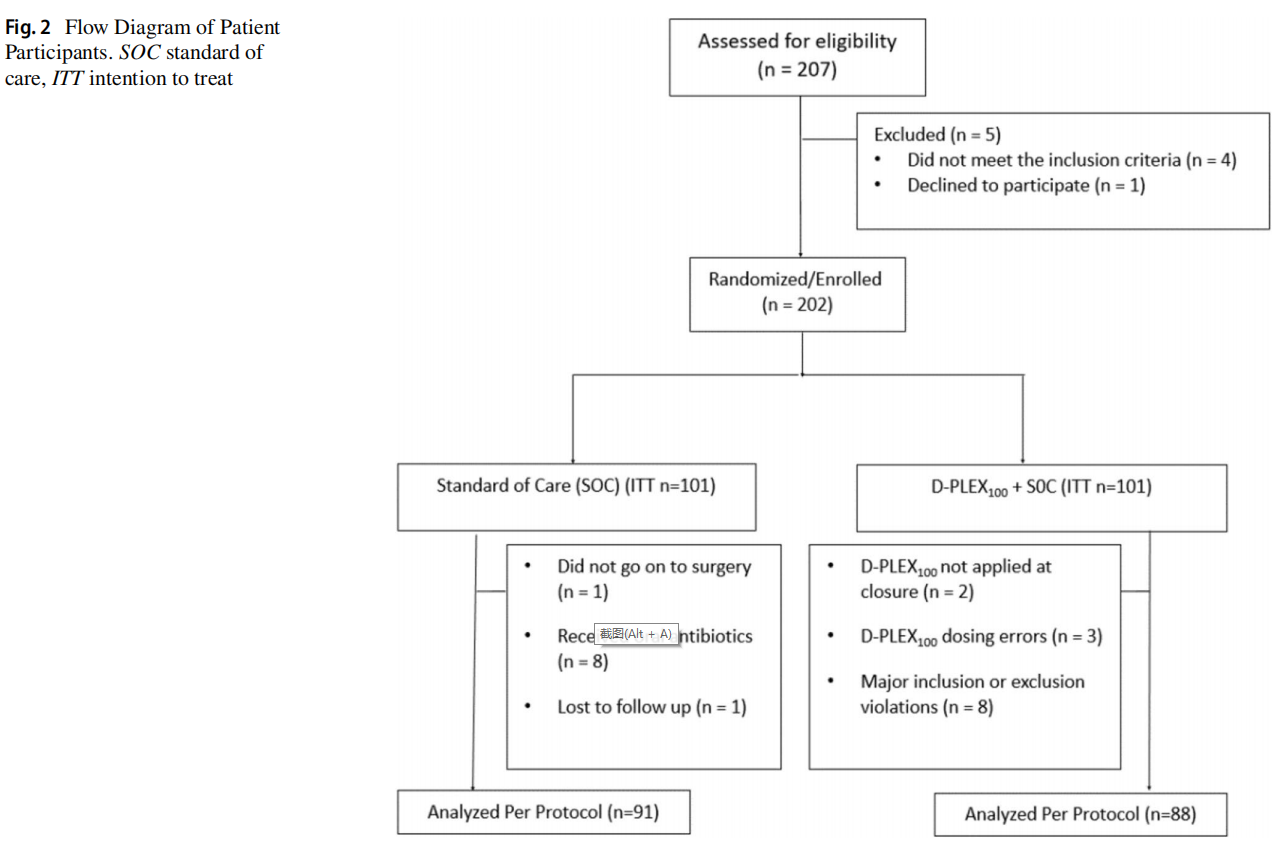
The groups were stratified based on patient demographics and surgical considerations as described in Table 1. There were no clinically meaningful differences in individual baseline characteristics between treatment arms. The only statistically significant difference between baseline characteristics was average patient height. Of note, the “Reason for Surgery” was not collected for 2 patients in the SOC arm. All surgical wounds were classified as clean-contaminated. All incisions were closed via primary intention.
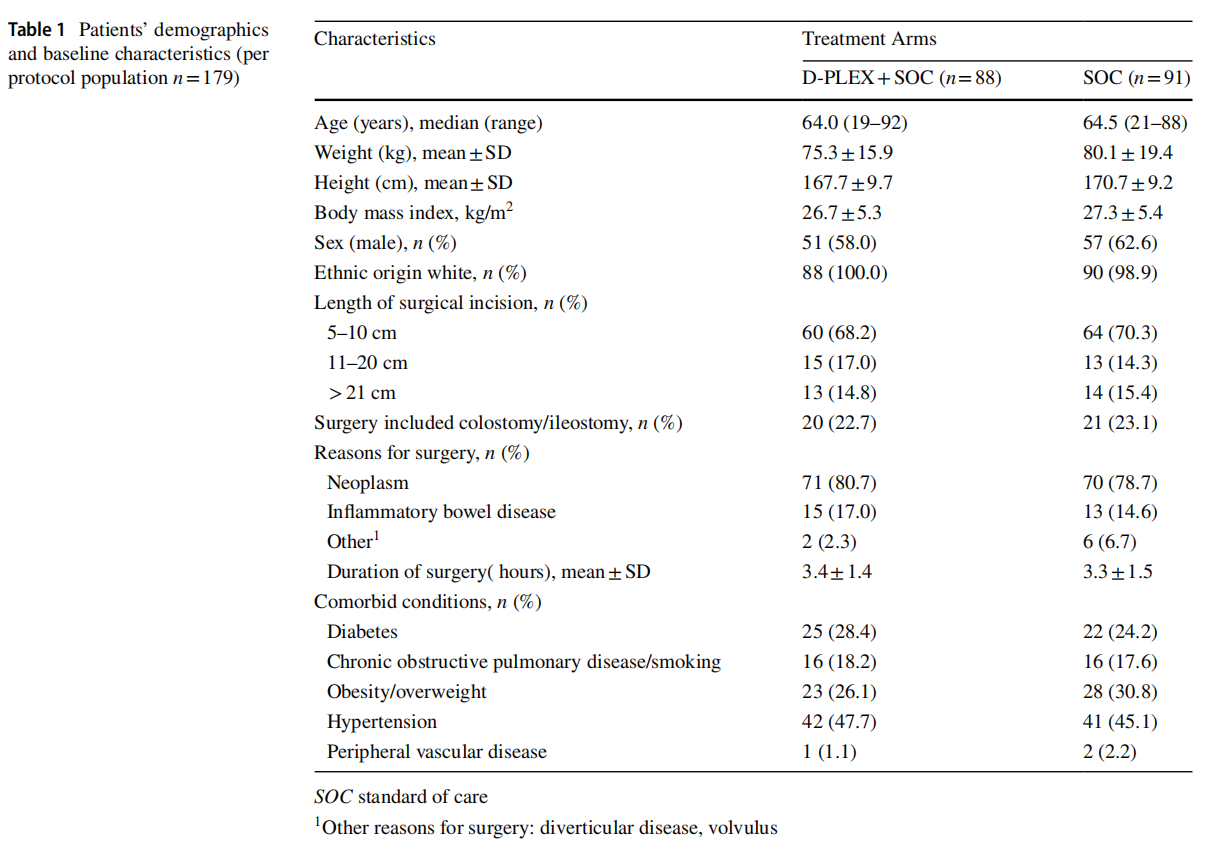
The rate of superficial and deep SSIs within 30 day post index surgery revealed a 64% relative risk reduction in the D-PLEX cohort [N=7/88 (8%)] vs. SOC [N=20/91 (22%)]; p=0.0115 (Table 2).
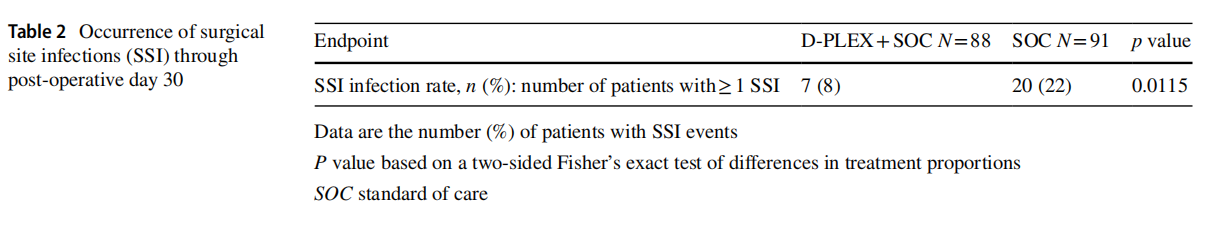
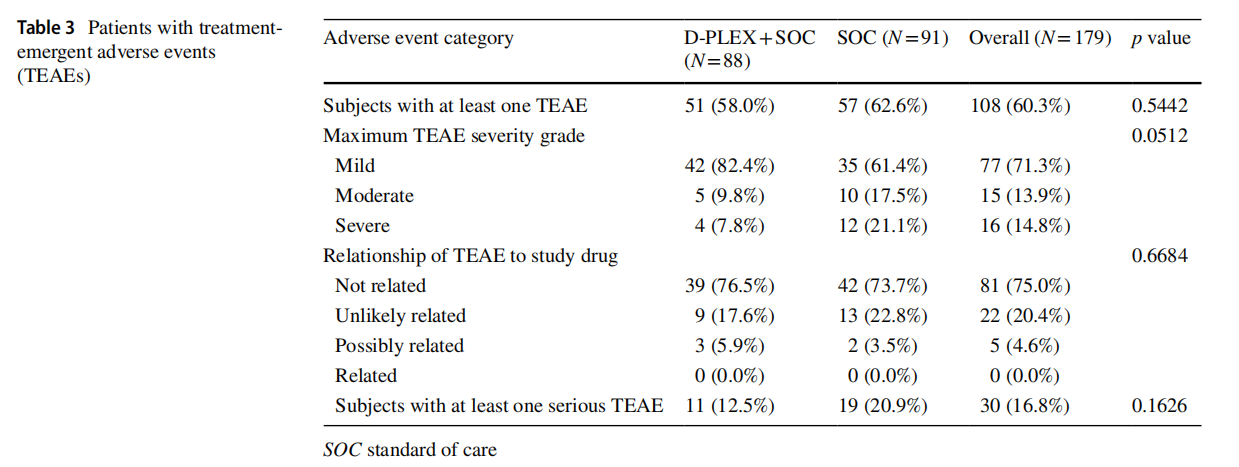
Table 3 summarizes the findings related to TEAEs. There was no statistically significant difference in incidence of TEAEs between the two groups. This includes differences in severity and incidence of serious TEAEs, although the D-PLEX cohort had a lower incidence of maximum TEAE severity which closely approached statistical significance. No TEAEs in the D-PLEX arm were deemed related to the study drug by the blinded assessor or endpoint adjudication committee. Interestingly, given that the assessors were blinded each patients’ randomization status, 15 patients (26.3%) in the SOC group had TEAEs which were deemed either “Unlikely” or “Possibly” related to the study drug (which the patients had not received).
Discussion
Even with the impressive advances in surgical technique and comprehensive strategies for perioperative infection reduction, superficial and deep SSIs remain a significant problem with major implications for patient outcomes, hospital metrics, and healthcare economics. Additionally, upwards of 50% of post-operative infections following colorectal surgery present outside of the hospital in the first 30 days [10], which may have substantial implications for post-operative care planning and validate the need for new, protective strategies against the development of these SSIs.
Our results show a statistically significant reduction in 30 day superficial and deep SSI incidence following D-PLEX administration without an associated increase in incidence or severity of adverse events.
It is important to consider that the safety and efficacy of D-PLEX was assessed in a setting that did not require MBP and specifically excluded OABP. While many surgeries requiring abdominal soft-tissue incisions such as urologic, gynecologic, and hepatobiliary procedures rely solely on intravenous antibiotics for systemic prophylaxis, the perceived rationale for inclusion of OABP and MBP in colorectal surgery is to reduce the potential soft-tissue wound contamination from bacteria originating in the lumen of the manipulated open bowel during the operative procedure [11–15]. Multiple clinical studies and meta-analyses have shown that MBP alone does not reduce the incidence of superficial or deep SSIs, and much of the recent literature seems to coalesce on the use of oral antibiotic prophylaxis in combination with intravenous antibiotic prophylaxis and/ or mechanical bowel preparation [11, 16–20]. Despite this, there are still studies which conclude that there is no difference in outcomes with bowel preparation compared to no bowel preparation [15], even while there is ongoing disagreement on the most appropriate OABP regimen [16] and many developed countries do not routinely include OABP [21]. Given the debate, the data presented here may offer guidance on the potential role for D-PLEX in cases where bowel preparation is contraindicated, procedures for Class 2, 3, or 4 wounds, or in cases of unanticipated bowel resection to improve SSI prophylaxis in soft-tissue (myofascial layer, subcutaneous fat, and skin) incisions.
Multiple studies have evaluated the efficacy of locally applied antibiotic agents to the abdominal surgical wound with varying results [22]. The use of a gentamicin-collagen sponge placement following colorectal surgery was evaluated in a multi-center Phase 3 clinical trial and showed no SSI reduction compared to SOC [23]. More recently, a Phase 2b trial studied the use of a bioresorbable, modified-release gel containing a combination of vancomycin and gentamicin also failed to demonstrate a significant benefit in SSI reduction [24]. Of the postulated reasons for these findings, the authors note the need for “rethinking of the proposed drug formulation.” The study notes the duration of the gel at approximately 48 h, which is remarkably shorter than the roughly 700 h duration for D-PLEX [9]. While formally planned studies are certainly necessary for further validation, it is interesting to postulate the utility of a prolonged-eluted locally applied antibiotic treatment option such as D-PLEX as an addition to current standard prophylactic recommendations.
There were limitations in this study which deserve consideration. Given that nearly 100% of the study population identified as ethnically white, our sample population is uniformly homogeneous and not representative of a more diverse patient population. Since certain populations, particularly those of African descent, have a well-described higher propensity for keloid formation and may be more susceptible to adverse post-operative hypertrophic scar formation [25, 26], studies involving more diverse patient populations are required to ensure the consistent safety profile demonstrated in this study. While the SOC SSI rate of 22% may initially appear high, it is within commonly reported ranges in colorectal surgery [17–20,27, 28]. Additionally, while it may not have had yielded any meaningful differences, other post-operative data points, such as glucose levels and body temperature, would have been important to collect and evaluate, as many of the current SSI bundle recommendations include maintaining euglycemia and normothermia [4, 27]. Although the association of SSIs with laparotomy incision type, extraction incision location, and conversion from a minimally invasive approach to open surgery are areas of clinical interest, the sample size precludes any meaningful evaluation of these relationships in this study. Although it is unlikely that MBP had a meaningful effect on SSI rate, as discussed above, since this aspect of prophylaxis was not standardized, there may be tangential biases not accounted for in the study design, and even with considerations provided earlier in the discussion, many audiences, particularly in the United States, expect the evaluation of SSI rates to involve the use of OABP and MBP and, therefore, warrant consideration in future studies.
Conclusions
The application of D-PLEX100 to the incisional wound bed following myofascial closure provided a statistically significant and clinically meaningful decrease in superficial and deep SSI rates without an increase in TEAEs. As such, it is a promising addition to current SSI prophylaxis bundle recommendations and is currently under evaluation in two Phase 3 clinical trials.
Acknowledgements To the Principal Investigators participating in this study: Katia Dayan, MD, Wolfson Medical Center, Holon, Israel, Shmuel Avital, MD, Meir Medical Center, Kfar Saba, Israel, Guy Lin, MD, Kaplan Medical Center, Rehovot, Israel, Sergio Susmallian, MD, Assuta Ramat-Hachayal, Tel Aviv, Israel; to Iris Gelber, MS, for the clinical study operation; to Tzvia Selzer, PhD, for critical review of the manuscript; to Eli Eyal, MSc, for review of the statistical analyses and the manuscript. Statistical analysis support was provided by Scientific and Medical Affairs Consulting, LLC.
Funding Research funding support was provided by PolyPid, Ltd.
Declarations
Conflict of interest O.Z. is an ad hoc speaker for PolyPid, Ltd.; Y.S., O.B., M.R., G.K., A.S., and N.E. are all employees of PolyPid, Ltd. or PolyPid, Inc. and own stock/stock options; N.W., H.T., and L.S. declare they have no conflicts of interest.
Ethical approval All procedures performed in this study were reviewed and approved by the institutional review board at each participating site and in accordance with the principles of Good Clinical Practice, the 1964 Declaration of Helsinki and its later amendments, and the International Council of Harmonization guidelines, as well as local
Informed consent All patients provided written informed consent prior to any study procedures.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4. 0/
References
1. Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA (2020) Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National healthcare safety network, 2015–2017. Infect Control Hosp Epidemiol 41(1):1–18. https://doi.org/10.1017/ ice.2019.296 (Epub 2019 Nov 26 PMID: 31767041 PMCID: PMC8276252)
2. Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, Tsai TC, Ko CY, Bilimoria KY (2015) Underlying reasons associated with hospital readmission following surgery in the United States. JAMA 313(5):483–495. https://doi.org/10.1001/ jama.2014.18614 (PMID: 25647204)
3. Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ (2011) Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 32(2):101–74. https://doi.org/10.1086/657912 (PMID: 21460463)
4. Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, Itani KM, Dellinger EP, Ko CY, Duane TM (2017) American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J Am Coll Surg 224(1):59–74. https://doi.org/10.1016/j.jamco llsurg.2016.10.029 (Epub 2016 Nov 30 PMID: 27915053)
5. Sheikh S, Malik NK, BAKarim (2020) Antibiotic prophylaxis and surgical site infections; a prospective open label study to clinically evaluate the serum and tissue concentration of single dose prophylactic ceftriaxone in laparoscopic cholecystectomy. Eur J Clin Pharmacol. https://doi.org/10.1007/s00228-020-02940-x
6. Takayama Y, Komatsu T, Nakamura T, Tomoda Y, Toda M, Miura H, Sato T, Atsuda K, Okamoto H, Hanaki H (2021) Association of serum and fat tissue antibiotic concentrations with surgical site infections in lower gastrointestinal surgery. Surgery S0039– 6060(21):00973–00979. https://doi.org/10.1016/j.surg.2021.10. 013 (Epub ahead of print. PMID: 34772516)
7. Toma O, Suntrup P, Stefanescu A, London A, Mutch M, Kharasch E (2011) Pharmacokinetics and tissue penetration of cefoxitin in obesity: implications for risk of surgical site infection. Anesth Analg 113(4):730–737. https://doi.org/10.1213/ANE.0b013e3182 1ff74 (Epub 2011 Jun 3 PMID: 21642605)
8. Metsemakers WJ et al (2015) A doxycycline-loaded polymer-lipid encapsulation matrix coating for the prevention of implant-related osteomyelitis due to doxycycline-resistant methicillin-resistant Staphylococcus aureus. J Control Release 10(209):47–56. https:// doi.org/10.1016/j.jconr el.2015.04.022
9. Kachel E, Card Surg J et al (2020) Local prolonged release of antibiotic for prevention of sternal wound infections postcardiac surgery—A novel technology. J Cardiac Surg. 35(10):2695–2703. https://doi.org/10.1111/jocs. 14890 (Epub 2020 Aug 2. PMID: 32743813)
10. Graham LA, Wagner TH, Sambare TD, Hawn MT (2021) Timing and cost of wound complications after colorectal resection. Dis Colon Rec 64(12):1551–1558
11. Bratzler DW, Dellinger EP, Olsen KM et al (2013) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst Pharm 70:195–283
12. ACOG Practice Bulletin No (2018) 195: prevention of infection after gynecologic procedures. Obstet Gynecol 131(6):e172– e189. https://doi.org/10.1097/AOG.0000000000002670 (PMID: 29794678)
13. Fujino Y (2015) Perioperative management of distal pancreatectomy. World J Gastroenterol 21(11):3166–3169
14. Ceppa EP, Pitt HA, House MG, Kilbane EM, Nakeeb A, Schmidt CM, Zyromski NJ, Lillemoe KD (2013) Reducing surgical site infections in hepatopancreatobiliary surgery. HPB (Oxford)
15(5):384–391. https://doi.org/10.1111/j.1477-2574.2012.00604.x (Epub 2012 Nov 5. PMID: 23557410; PMCID: PMC3633041)
15. Koskenvuo L, Lehtonen T, Koskensalo S, Rasilainen S, Klintrup K, Ehrlich A, Pinta T, Scheinin T, Sallinen V (2019) Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 394(10201):840–848. https://doi. org/10.1016/S0140-6736(19)31269-3 (Epub 2019 Aug 8 PMID: 31402112)
16. Ju YU, Min BW (2021) A review of bowel preparation before colorectal surgery. Ann Coloproctol. 37(2):75–84. https://doi.org/ 10.3393/ac.2020.04.01 (Epub 2020 May 15. PMID: 32674551; PMCID: PMC8134921)
17. Klinger AL, Green H, Monlezun DJ, Beck D, Kann B, Vargas HD, Whitlow C, Margolin D (2019) The role of bowel preparation in colorectal surgery: results of the 2012–2015 ACS-NSQIP data. Ann Surg 269(4):671–677. https://doi.org/10.1097/SLA.00000 00000002568 (PMID: 29064902)
18. McSorley ST, Steele CW, McMahon AJ (2018) Meta-analysis of oral antibiotics, in combination with preoperative intravenous antibiotics and mechanical bowel preparation the day before surgery, compared with intravenous antibiotics and mechanical bowel preparation alone to reduce surgical-site infections in elective colorectal surgery. BJS Open 2(4):185–194. https://doi.org/ 10.1002/bjs5.68 (PMID:30079387 PMCID:PMC6069350)
19. Ohman KA, Wan L, Guthrie T, Johnston B, Leinicke JA, Glasgow SC, Hunt SR, Mutch MG, Wise PE, Silviera ML (2017) Combination of oral antibiotics and mechanical bowel preparation reduces surgical site infection in colorectal surgery. J Am Coll Surg 225(4):465–471. https://doi.org/10.1016/j.jamco llsurg.2017. 06.011 (Epub 2017 Jul 6 PMID: 28690206)
20. Van’t Sant HP, Weidema WF, Hop WC, Oostvogel HJ, Contant CM (2010) The influence of mechanical bowel preparation in elective lower colorectal surgery. Ann Surg 251(1):59–63. https:// doi.org/10.1097/SLA.0b013e3181c0e75c (PMID: 20009750)
21. Battersby CLF, Battersby NJ, Slade DAJ, Soop M, Walsh CJ (2019) Preoperative mechanical and oral antibiotic bowel preparation to reduce infectious complications of colorectal surgery- the need for updated guidelines. J Hosp Infect 101(3):295–299. https://doi.org/10.1016/j.jhin. 2018.12.010 (Epub 2018 Dec 21 PMID: 30579970)
22. Nelson RL, Iqbal NM, Kravets A, Khateeb R, Raza M, Siddiqui M, Taha I, Tummala A, Epple R, Huang S, Wen M (2018) Topical antimicrobial prophylaxis in colorectal surgery for the prevention of surgical wound infection: a systematic review and meta-analysis. Tech Coloproctol 22(8):573–587. https://doi.org/ 10.1007/s10151-018-1814-1 (Epub 2018 Jul 17. Erratum in: Tech Coloproctol 2019 Apr 1: PMID: 30019145)
23. Bennett-Guerrero E, Pappas TN, Koltun WA, Fleshman JW, Lin M, Garg J, Mark DB, Marcet JE, Remzi FH, George VV, Newland K, Corey GR. SWIPE 2 Trial Group (2010) Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N Engl J Med 363(11):1038–1049 (PMID: 20825316)
24. Bennett-Guerrero E, Berry SM, Bergese SD, Fleshner PR, Minkowitz HS, Segura-Vasi AM, Itani KMF, Henderson KW, Rackowski FP, Aberle LH, Stryjewski ME, Corey GR, Allenby KS (2017) A randomized, blinded, multicenter trial of a gentamicin vancomycin gel (DFA-02) in patients undergoing abdominal surgery. Am J Surg 213(6):1003–1009. https://doi.org/10.1016/j. amjsurg.2016.10.007 (Epub 2016 Nov 18 PMID: 27989501)
25. Glass DA 2nd (2017) Current Understanding of the genetic causes of keloid formation. J Investig Dermatol Symp Proc 18(2):S50–S53. https://doi.org/10.1016/j.jisp. 2016.10.024 (PMID: 28941494)
26. Sun LM, Wang KH, Lee YC (2014) Keloid incidence in Asian people and its comorbidity with other fibrosis-related diseases: a nationwide population-based study. Arch Dermatol Res 306(9):803–808. https://doi.org/10.1007/s00403-014-1491-5 (Epub 2014 Aug 1 PMID: 25081927)
27. Meeks DW, Lally KP, Carrick MM, Lew DF, Thomas EJ, Doyle PD, Kao LS (2011) Compliance with guidelines to prevent surgical site infections: as simple as 1–2-3? Am J Surg 201(1):76–83. https://doi.org/10.1016/j.amjsurg.2009.07.050 (Epub 2010 Jun 22 PMID: 20573335)
28. Lawson EH, Hall BL, Ko CY (2013) Risk factors for superficial vs. deep/organ-space surgical site infections: implications for quality improvement initiatives. JAMA Surg 148(9):849–858. https://doi.org/10.1001/jamas urg.2013.2925
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the Techniques in Coloproctology (2023) 27:209–215 by Wound World.


