1、Introduction
Surgical site infections (SSI) are the leading cause of hospital readmission after surgical procedures [1] with significant impact on post-operative morbidity and mortality [2] Modifiable risk factors for SSI include procedural aspects, which include the possibility of instrument contamination, the duration of the operation, the number of people present and the traffic in the room [3], and the ventilation system of the operating theatre [4].
These factors influence each other because preparation of instrumentation involves processes that involve a certain number of operators, each step increases the risk in contamination, and the need for different instrumentation, depending on the type of operation, can lead to altered traffic in theatre.
The need to optimise infection control in OT is accentuated by the SARS-CoV-2 pandemic and the need to prevent and respond to future epidemics.
1.1 Research questions
1) What is the relationship between the features of surgical procedure sets and the frequency of surgical site infections (SSI) in patients undergoing surgical treatment?
2) How do the time frames of perioperative processes and operating theatre traffic vary in relation to the features of the procedure sets used?
3) What is the impact of streamlining and optimising surgical procedure sets and their direct and indirect costs?
2、Methods
This systematic review is conducted in accordance with the Preferred Reporting Items for Systematic Reviews and MetaAnalyses—PRISMA Statement 2020 [5].
Accordingly, the literature review process was carried out in the following stages:
1. Formulation of research questions by adapting the PICOS model
2. Developing a search string applied to databases;
3. Collection of identifed records;
4. Screening of records according to inclusion criteria;
5. Full-text selection of the studies identifed during screening;
6. Data extraction from the studies included;
7. Thematic analysis of results
2.1 Research strategy
The search was applied to the following databases: MEDLINE, Embase, Web of Science, CINAHL, The Cochrane Library.
The search string was formed by combining the following terms:
1. Procedure or procedural or surgery or surgical.
2. Tray or pack.
3. 1 and 2
The complete search strategy can be found in Appendix 1.
2.2 Selection of studies
An initial screening was performed on the title and abstracts of the Records identified in the databases. Subsequently, the selected records were analysed on full text for inclusion or exclusion. The selection was made according to the following criteria:
Participants: surgical specialists, operating theatre (OT) nurses and nurse coordinators, theatre technicians, sterilisation centre (SC) coordinators and operators, other personnel with clinical, organisational, or logistical roles; patients undergoing surgical treatment
Intervention: use of conventional or disposable procedure sets; processes of rationalising surgical sets
Outcome: incidence of SSIs, procedure time, in/out traffic of personnel, costs related to surgical procedures
Setting: general and specialist surgery facilities, supply services and sterilisation centres (SC)
Study designs: qualitative, observational, nRCTs and RCTs, systematic reviews, guidelines
English-language studies published up to 28.12.2021 were selected.
Any previous guidelines, systematic reviews or meta-analyses discovered during the database search, concerning surgical sets from the point of view of clinical risk reduction, procedure optimisation and the rationalising of instruments, were selected and summarised in a separate table.
2.3 Data extraction
The data are extracted from the studies in a specially designed worksheet. Data were extracted on the test population, the surgical setting, the procedures performed, the operations and any follow-ups. The main operational outcomes of interest were:
1. A reduction in the incidence of post-surgical SSIs;
2. A reduction in procedure times;
3. A reduction in operating theatre traffic flow;
4. A reduction in costs associated with the intervention.
3、Results
Figure 1 represents the flow of the literature review effected. A search through PubMed, Embase, CINAHL, Web of Science and The Cochrane Library identified 5 865 records. Five further studies were added manually after consulting the bibliographies of the articles analysed. After eliminating duplicates, the remaining records were screened by title/abstract. 98 records were assessed as eligible and analysed on full text.
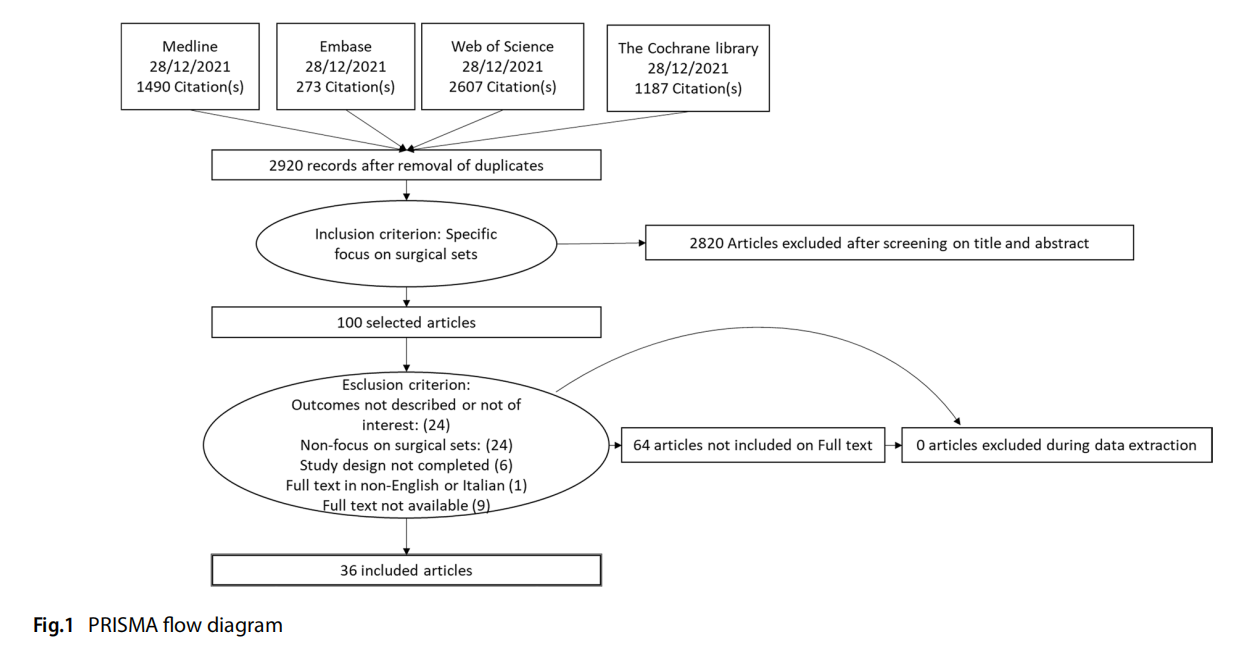
A total of 62 studies were excluded from the complete analysis. In conclusion, 36 studies were eligible for inclusion in the review.
A complete list of selected studies and the relevant bibliography can be found in Appendix 2.
Excluded, reasons for categorisation: > 62:
– full text not available (9): Littell 1951, Sebben 1988, Sheth 2003, Dieryck 1998, Stephanie 2010, Akridge 2004, Osborne 1999, Passey 2002, Wilkie 1986
– Outcome not described or not of interest: (24) Ahmadi, 2019; Egan 2021, Baskett 2004, Bhumisorikul 2004, Costa 2018, Fogliatto 2018, Glaser 2015, Halbert 1988, Huang 2021, Kimse 2021, Kusuda 2016, Strulak 2021; Strzelecki, 1989, Torres 2021, Bradley 2019, Alfred 2021, Dana Barlow 2015, Holdsworth 2021, Igesund 2019, Navi 2012, Parker 2006, Wells 2017, Wells 2018, Eggleston 1997
– No focus on surgical sets: (24) Agarwal 2019, Arslan 2018, Dilworth 1992, Edilich 1992, Fadaak 2021, Haya 2018, Johnson 2016, Kaygusuz 2003, Khurana 2018, Kong 1994; Kwaan 2016; Lin 2018, Martinez 2020; McDermott 2016; Meals 2007; Moccia 2020; Nguyen 2019; Panahi 2012; Rao 1992, Wagner 2021, Weiser 2018; Watters, 2011; Makram 2021, Olivere 2021
– Study design not covered (e.g. editorials, case reports, opinion papers) (6): Reams 2013, Weber 1998, Nadeau 2018, O’Donnell 2002, Stephanie 2010, Goldberg 2019
– Full text in other language (1): Blanc 2017
Including: 36:
– Previous reviews on the subject (4)
– Primary studies (32)
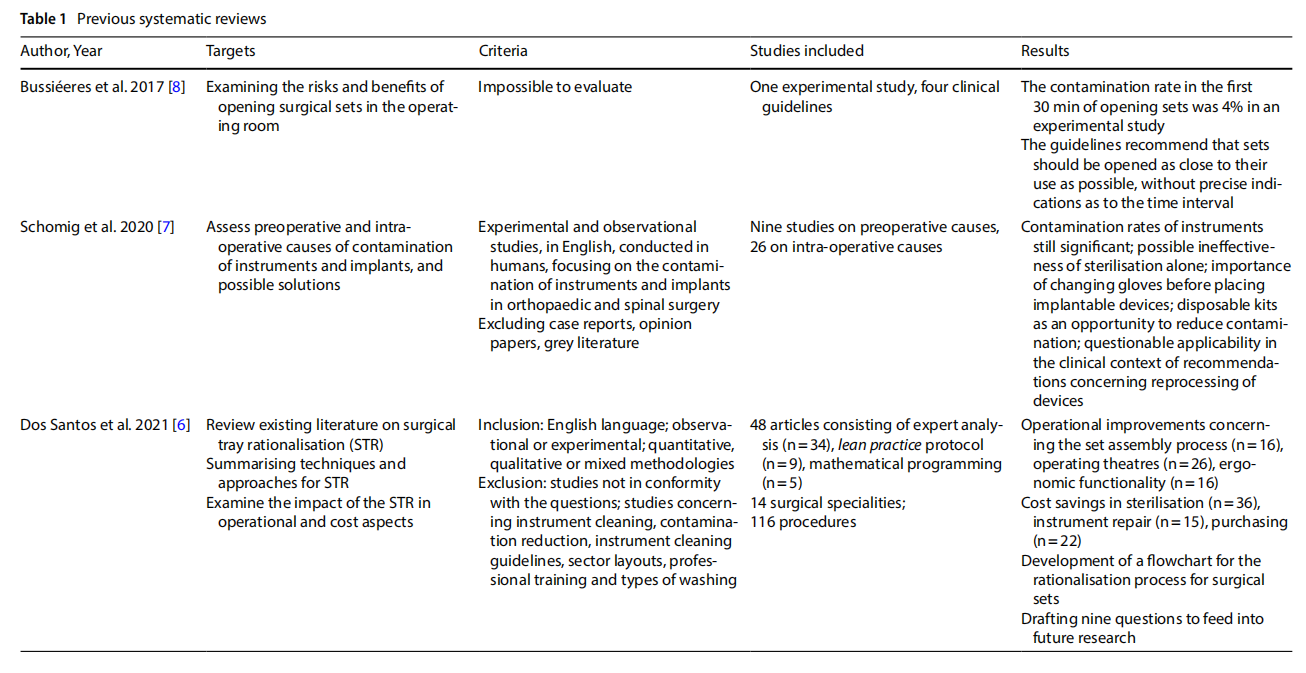
3.1 Summary of evidence prior to current review
Four literature reviews relevant to the scope of the current work were identified (Table 1). In particular, Dos Santos and colleagues [6] sought to understand the main techniques and approaches in the rationalising of surgical sets, the impact on financial and operational performance, and the knowledge gaps towards which research should be directed. The review identified many studies reporting signs of improved performance both operationally, concerning set assembly, OR management and ergonomics, and efficiently in terms of washing and sterilisation processes, repair, purchasing, set-up and professional education. The authors mapped an outline for future research. Salient points to be explored are: the promotion of consensus building in multidisciplinary teams, participation in surgical set rationalisation projects and consolidation of the progress achieved; technologies for instrument tracking; cross-surgical set analysis aimed at instrument reduction; relocation of instruments excluded from sets; how to measure “non-material” and “non-tangible” benefits of set rationalisation and set safety after the process; objectives following the establishment of a surgical set rationalisation cycle.
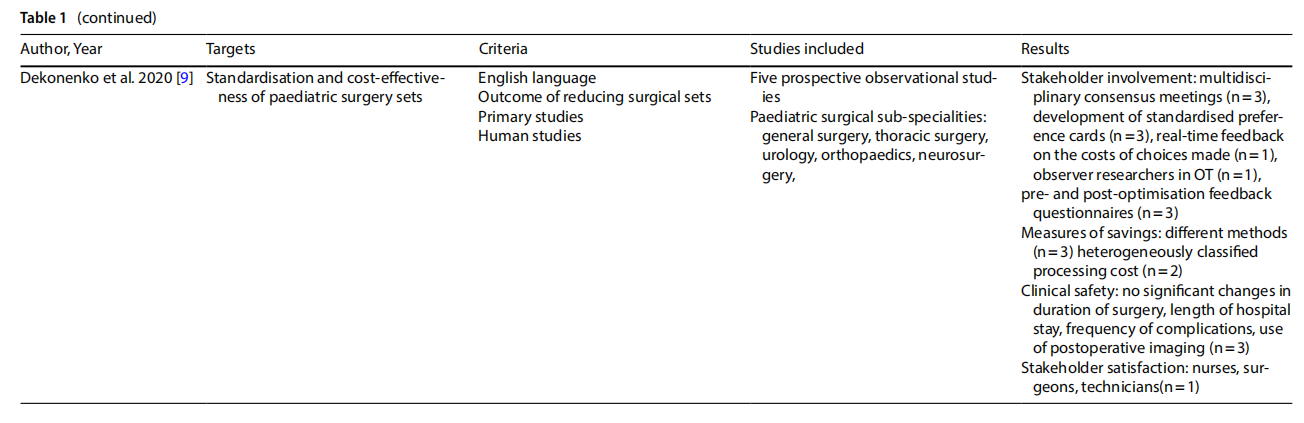 A systematic review [7] analysed the problem of contamination of implantable instruments and devices in spinal surgery. Many studies have shown significant contamination rates. Preoperative factors, mainly related to sterilisation and device handling processes, and intra-operative factors, more dependent on personnel procedures, were noted. With respect to pre-operative factors, several studies question the overall effectiveness of sterilisation alone. At the same time, recommendations for device reprocessing are recognised as being difficult to implement in clinical settings. Regarding intra-operative processes, studies emphasise the importance of preventive practices such as changing gloves before handling implant materials, because the risk of contamination increases with exposure time [7].
A systematic review [7] analysed the problem of contamination of implantable instruments and devices in spinal surgery. Many studies have shown significant contamination rates. Preoperative factors, mainly related to sterilisation and device handling processes, and intra-operative factors, more dependent on personnel procedures, were noted. With respect to pre-operative factors, several studies question the overall effectiveness of sterilisation alone. At the same time, recommendations for device reprocessing are recognised as being difficult to implement in clinical settings. Regarding intra-operative processes, studies emphasise the importance of preventive practices such as changing gloves before handling implant materials, because the risk of contamination increases with exposure time [7].
3.2 Clinical interventions to prevent surgical site infections
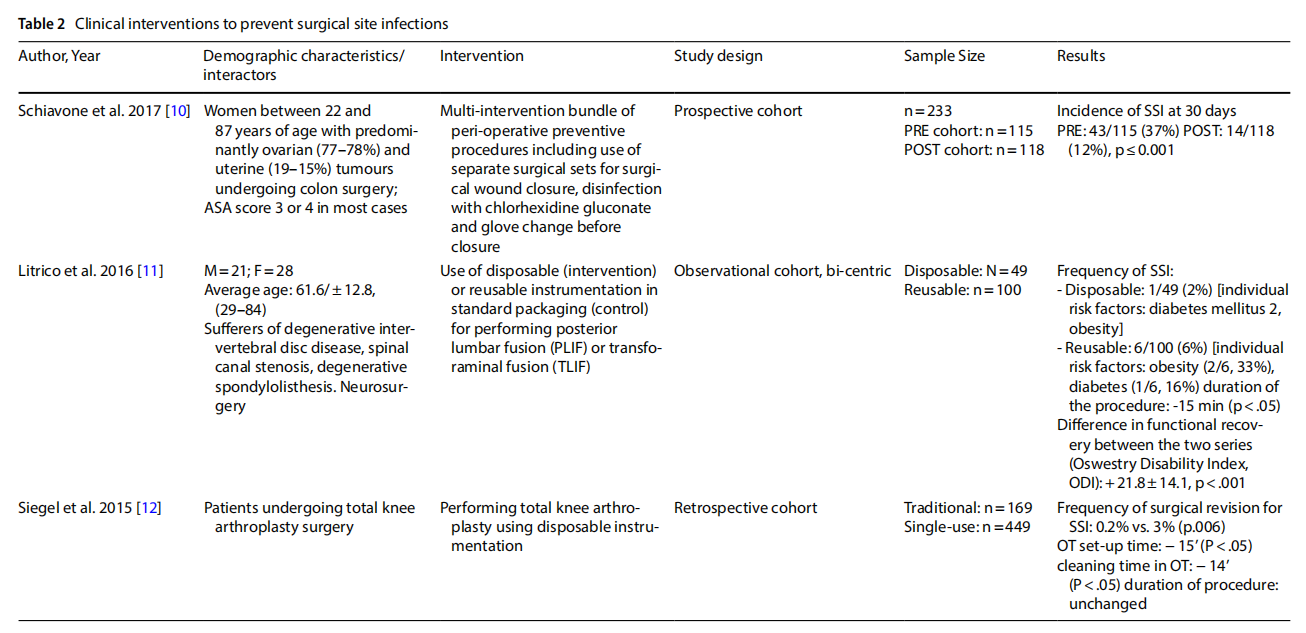
Three observational studies report direct evidence of an effect on the incidence of SSI (Table 2). A cohort study conducted on 233 women with ovarian and uterine neoplasms who underwent colon surgery showed a statistically significant reduction in SSIs 30 days after surgery after implementation of a preventive bundle targeting the surgical wound closure phase, including the use of a separate surgical set [10]. Two other observational studies investigated the use of disposable sets versus traditional reusable sets. The first, conducted on neuro-surgical patients undergoing lumbar fusion, observed a reduction in the incidence of SSIs from 6% in the series of 100 patients treated with reusable instruments to 2% in the series of 49 patients treated with disposable sets (p<0.001). A significant reduction in surgical procedure duration (p<0.05) and functional recovery time (p<0.001) was also observed [11]. In the second, 449 orthopaedic patients underwent total knee arthroplasty using single-use instrumentation and only 0.2% underwent revision surgery for SSIs, compared to 3% (p<0.006) of cases in a series of 169 patients operated on with conventional instrumentation [12].
3.3 Procedures for surgical tray rationalisation
Twenty-nine surgical tray rationalisations (STR) as part of quality improvement projects aimed at creating an optimised set for specific procedures.
In this context, unused items were removed from the instrument management process.
The studies presented in the literature (Table 3) involved multiple branches, including: orthopaedics (n=6), gynaecology (n=4), ENT (n=6), thoracic surgery, endocrine surgery, paediatric surgery (n=2), neurosurgery, ophthalmology, vascular surgery, breast surgery (n=2), urology, general surgery (n=2), hand surgery, and plastic surgery. Several studies included an observation phase involving the preparation, use and reprocessing of the surgical sets between the operating theatre (OT) and the sterilisation centre (SC) (n=13).
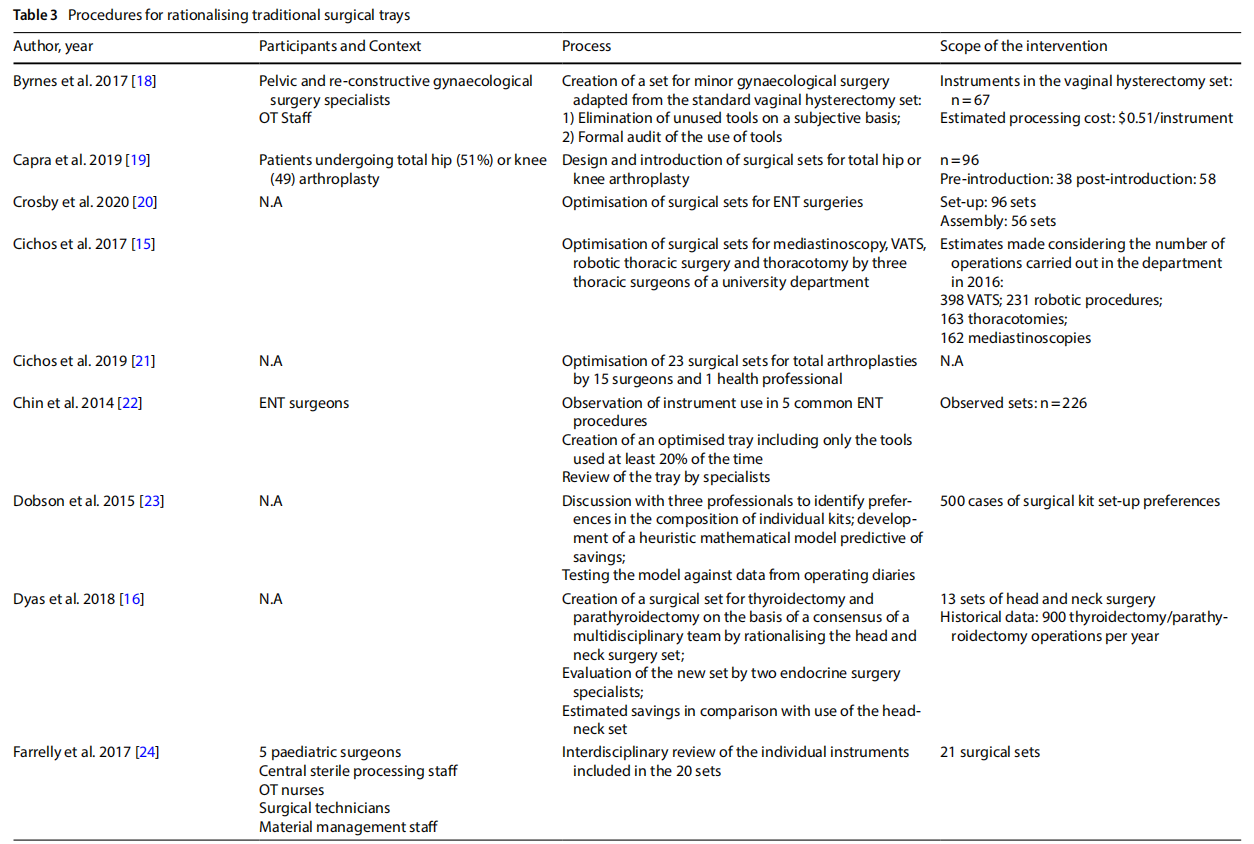
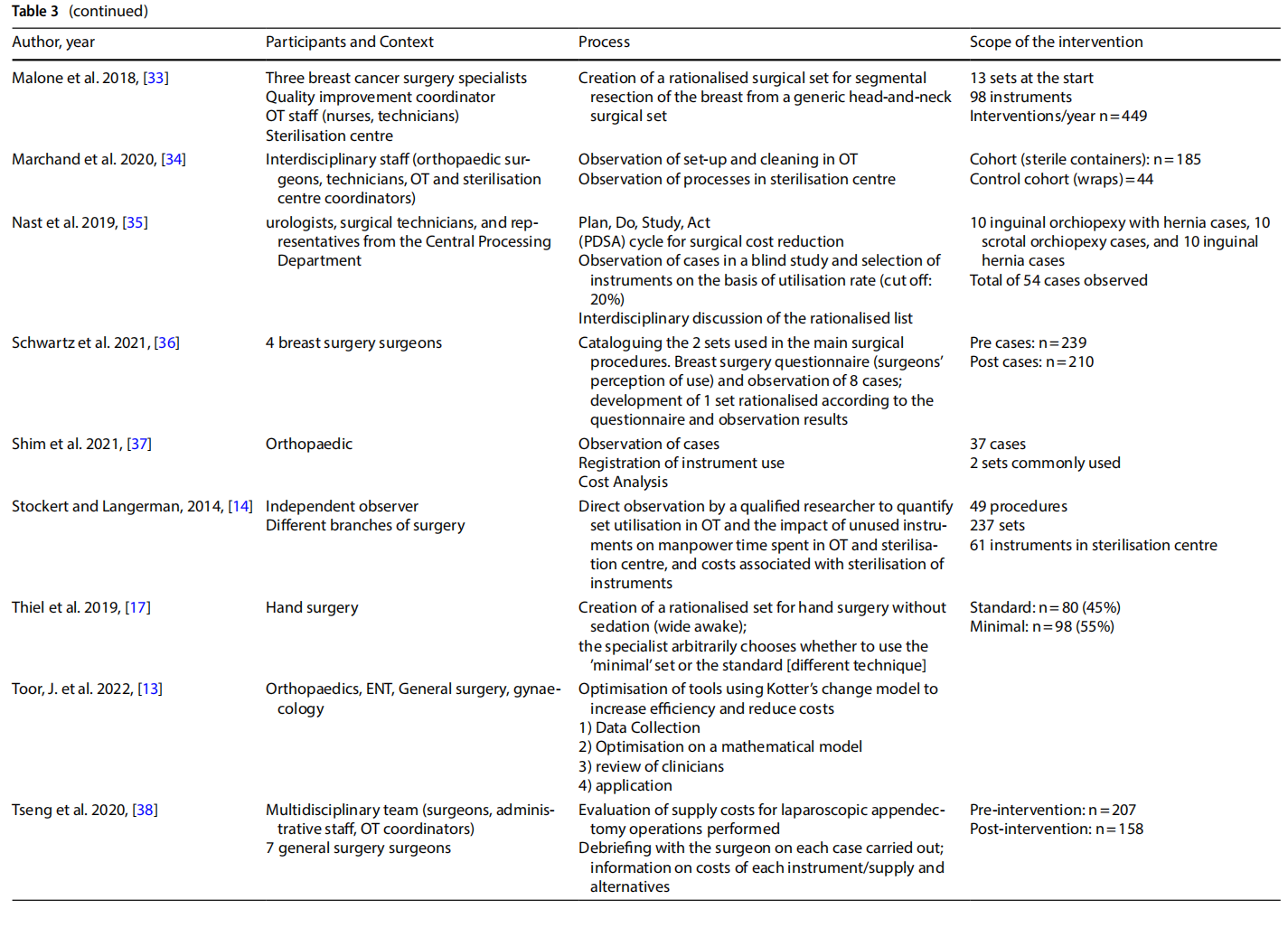
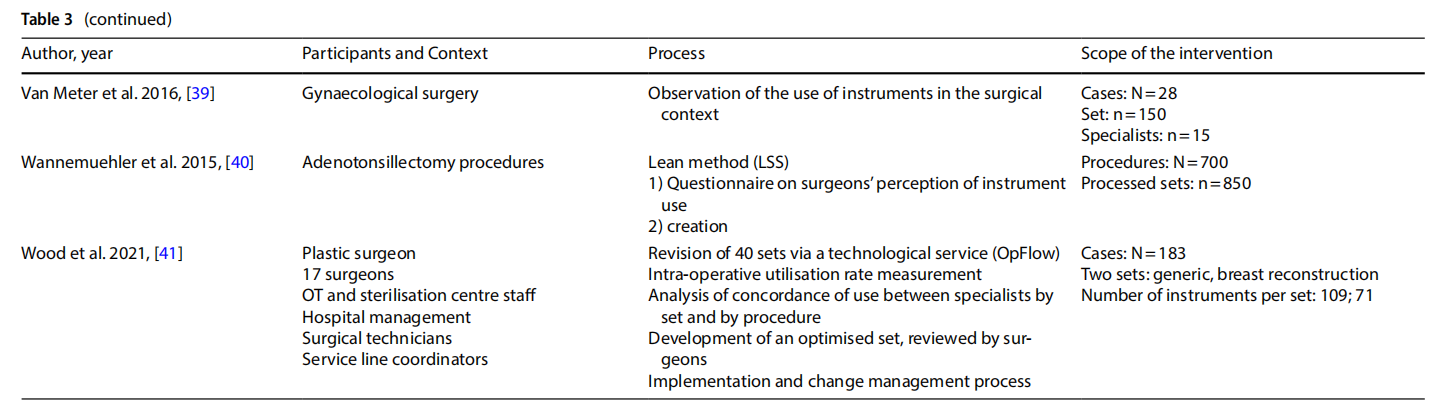
3.4 Processes
The rationalisation process was preceded by training and sessions to raise awareness (n=3) aimed at increasing the degree of motivation for change and overcoming resistance that was potentially risky for the entire process.
The extent to which an instrument is used, and therefore the benefits of keeping it in the set or not, was assessed on an objective basis by analysing the data collected during the observation (n =13), with the cut-of of use generally considered to be 20% to 25%. In other cases, the selection was made on a subjective basis by professionals (n =3), by consensus or the collection of questionnaires on perceived use. In one case a heuristic mathematical model was developed based on a discussion with skilled surgeons on their preferences as to the composition of individual sets [10]. The model was then tested on the operating division agendas with the goal of cost-cutting.
In some cases, only surgeons were involved (n = 6). In other cases, the formation of a multidisciplinary team was promoted (n=7).
In most of the studies, the new optimised set was presented to the clinicians for review. Devices or instruments were then added based on their opinion.
Surgical devices or instruments that were excluded were generally packaged in a dedicated set, or the original set remained available and the frequency of use of the instruments excluded was used as a marker of the safety of
Some studies considered a follow-up aimed at assessing the degree of satisfaction with the new rationalised set after a period of time (n=3).
3.5 Outcomes
The outcomes analysed were mainly of three types:
- reducing the size of the set and the number of instruments (Table 4);
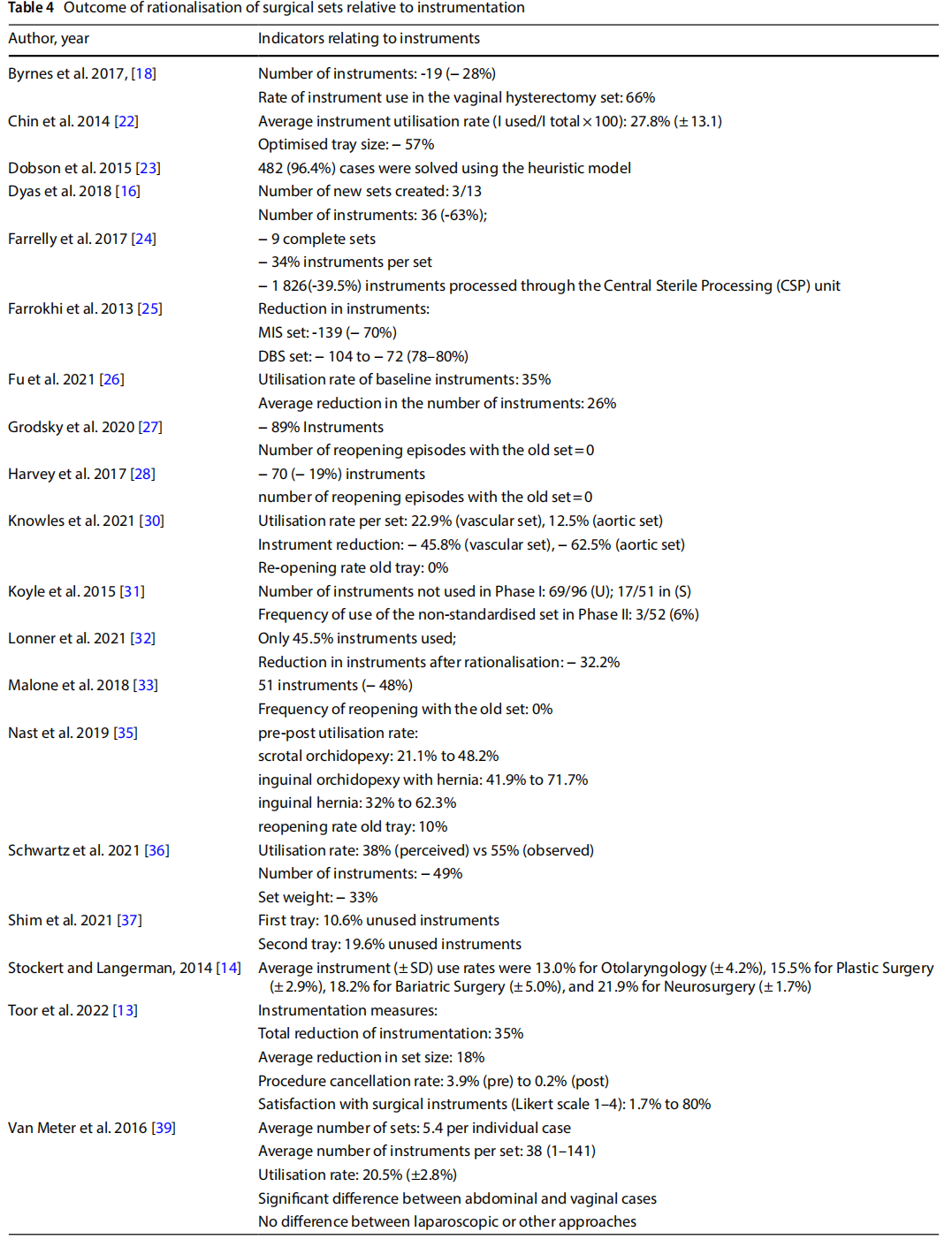
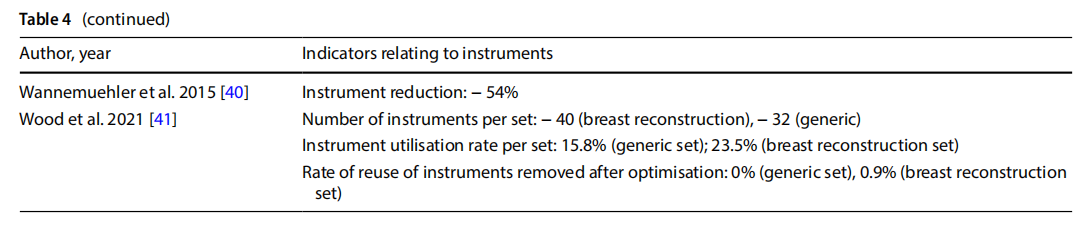
●reduction of peri-operative and sterilisation times and reprocessing of sets (Table 5);
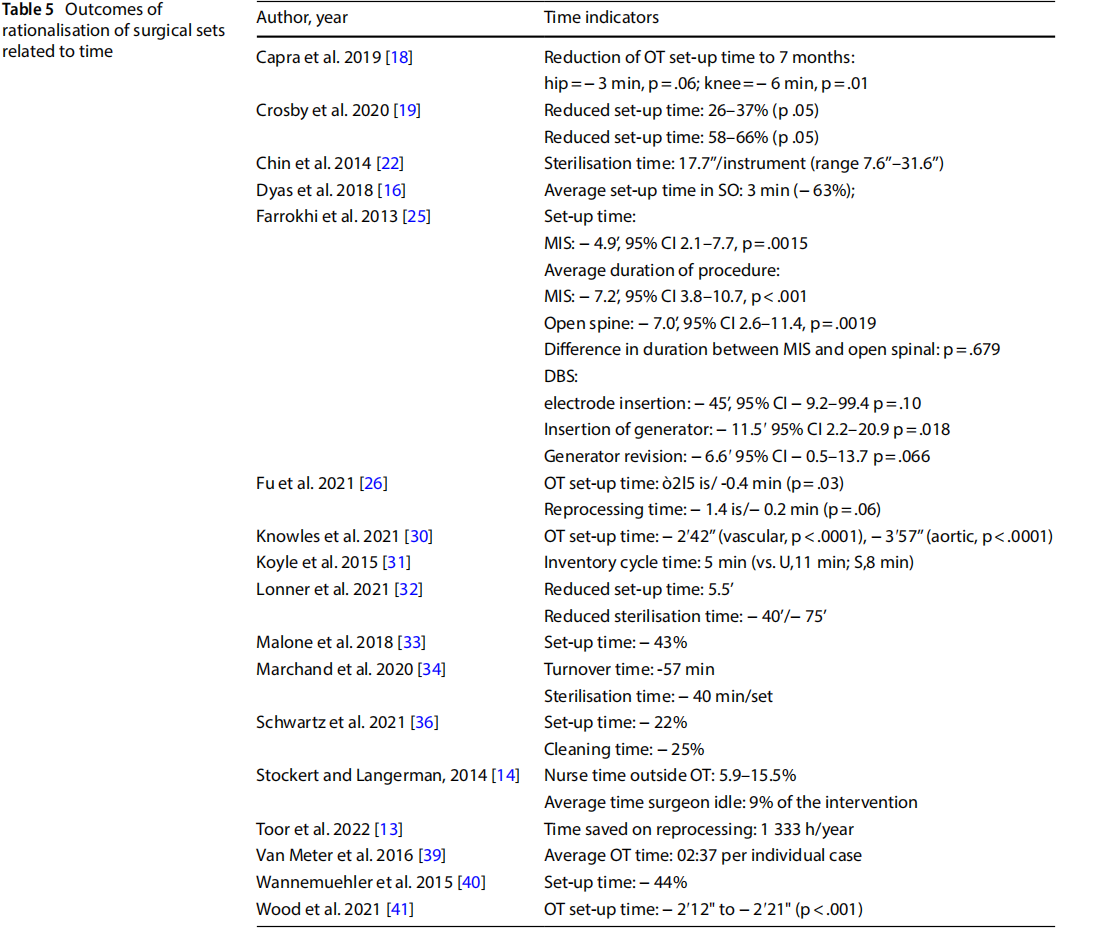
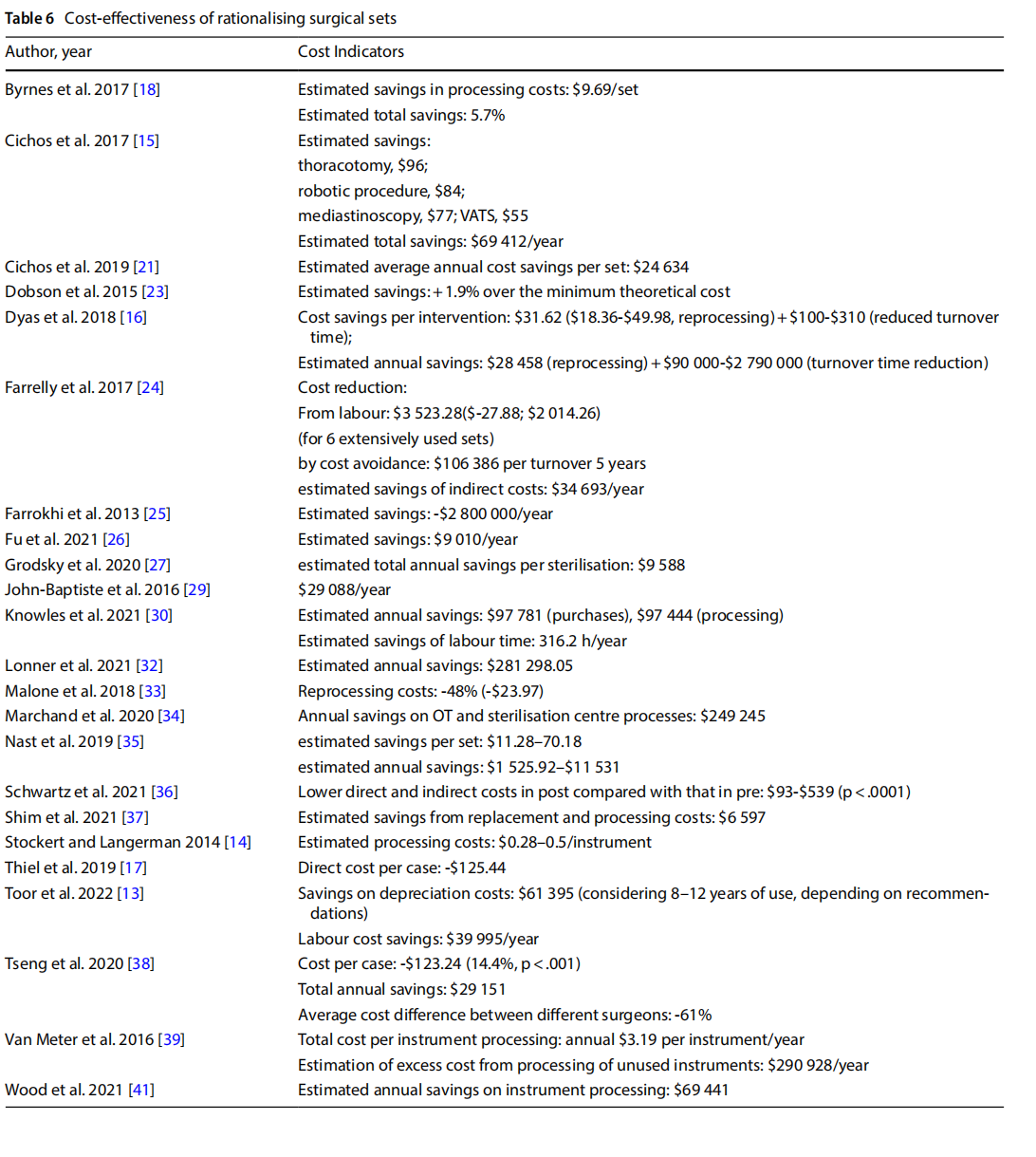
- reduction of direct and indirect costs related to the procurement, replacement and reprocessing of instrumentation (Table 6).
Therefore, the studies reported primary outcomes having an impact on the organisational framework.
18 studies indicated a reduction in the number of instruments from the original set to the optimised set. As five of these studies involved the optimisation of more than one set, they either reported a separate outcome for each set (n = 3) or an average value across sets (n = 2). Of the total number of items analysed, the percentage reduction in the number of instruments per individual set ranged from 19 to 89%.
In 10 studies, the observation phase in OT described the utilisation rate of the individual sets observed. Utilisation of instruments before optimisation ranged from 12.5% to 66%.
One study reported a comparison between the utilisation rate before and after the optimisation of two sets, showing an increase in use of between 27 and 30% [13].
One study analysed the diference between the perceived use of surgical devices and instruments by the surgeons involved and their actual use determined by observation, which were respectively 37% and 55% [12].
Seven studies measured the frequency of reopening the original set in a predetermined period after optimisation. Considering that a total of eight sets were optimised, in five instances no reopening events were recorded, in the other cases the frequency of reopening was 0.9%, 6% and 10% respectively.
One study measured a reduction in the frequency of procedure cancellations following the introduction of an optimised set: incidents dropped from 3.9% to 0.2% [16]. The same study submitted a questionnaire to gather indications of staff satisfaction with the instrumentation before and after optimisation, with an increase from 1.7% to 80% [13].
Eleven studies measured the time taken to set up the operating theatre, which decreased to 2 from 5 min following the introduction of optimised sets. Other studies observed that optimisation of the set had an impact on the overall duration of the procedure, with a 5 to 6-min reduction. A reduction in the time spent cleaning the operating theatre (-25%,n=1), and time spent by the nurse on duty outside the theatre for reasons related to retrieval of surgical instruments (-15.5%, n=1), were also observed. One study observed downtime of 9% of the entire procedure for reasons related to locating surgical instruments [14].
Concerning costs, many studies have estimated projected cost savings in the procurement, sterilisation and processing of instruments. The average annual savings were estimated at $1 525.00 to $2 800 000.00 (n=12), depending on the activity flow of the various hospitals involved.
Differences in estimated savings were observed depending on the differing procedures: from $55.00 to $310.00 per single procedure [15–17].
4、Discussion
The results of the systematic review brought to light observational studies that can be divided into two categories: evidence of purely clinical significance and evidence of mainly organisational, managerial, and financial significance. These two systems are largely interconnected, and reciprocally influence each other.
The decision to use disposable devices and instruments has been accompanied by a lower incidence in surgical site infections and surgical revisions for remediation. A concomitant reduction in post-operative functional recovery time has also been observed [11].
The rationalisation of traditional surgical sets has been observed in conjunction with outcomes of organisational significance, some of which could have an indirect clinical impact. As already highlighted by previous reviews, intraoperative time and time spent by nurses outside the room, as well as the reduction of setup time[7, 8] could lead to a further reduction in the time window of infection risk. Similarly, air movement in the operating room, potentially risky for contamination, could be reduced. However, studies reporting clinical outcomes as a direct consequence of organizational factors have not been found.
The effectiveness of the rationalisation of surgical sets seems to depend mainly on two conditions: the type of surgery, including the different access modes possible; and the organisational model followed. The main models observed in the interventions were Lean management and Kotter’s change model. Responsibility for a training and awareness-raising process, with the active involvement of all stakeholders, appears to play a decisive role in practitioners’ participation in rationalisation practices and the long-term maintenance of the results obtained.
4.1 Limits
The observational nature of all the studies identified means that the evaluations resulting from this review can be generalised taking into account the specific organisational context. This implies the need to promote future research on the topic by way of a context-specific analysis. In this context, research should consider the elements mentioned by Dos Santos and colleagues [6]: the promotion of increased consensus in multidisciplinary teams, participation in surgical set rationalisation projects and consolidation of the progress achieved; technologies for instrument tracking; a crosssectional analysis of surgical sets aimed at instrument reduction; relocation of instruments excluded from sets; how to measure the ’non-material’ and ’non-tangible’ benefits of rationalisation and set safety after the process; and objectives following the establishment of a surgical set rationalisation cycle. Furthermore, clinical practice could benefit from primary or secondary research design that can exemplify outputs as a result of modelling that includes an analysis of clinical or organisational improvement in relation to specifc organisational, cultural and functional frameworks. It should be noted that most of the quality improvement interventions analysed did not investigate the association with direct clinical outcomes on patients such as ISS incidence, mortality and morbidity, or the occurrence of other complications, due to the nature of the project, which is also exempt from ethical approval,due to the nature of the project which was also not accompanied by ethical approval. It is considered important that organisational process indicators go hand in hand in the future with an analysis of the direct effects in the clinical field, in order to further clarify the value of the processes considered.
Most of the studies were carried out in single centres. This implies, especially as regards rationalisation of surgical sets, that the results are extremely sensitive to the organisation of individual healthcare facilities and their catchment areas.
Cost estimates presented in studies have been developed retrospectively and in some cases according to direct caseby-case analysis. These projections may be sensitive to variables not examined, including the type and overall availability of instruments in the market, and may not be generalised regardless of the country in which the study was carried out.
Acknowledgements Thanks to Lorena Trivellato of BHAVE for her editorial assitance in drafting the manuscript.
Author contributions All authors have made substantial contribution to the design, acquisition and interpretation of data of the article. All authors read and approved the final manuscript.
Funding This work was supported by Monlycke Health Care Srl.
Data availability The datasets generated and analysed during the current study are not publicly available due, but are available from the corresponding author on reasonable request.
Declarations
Competing interests All authors report no confict of interests.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Appendices
Appendix 1: Comprehensive search strategies
MEDLINE
1. "Procedure"[Title/Abstract] OR "procedural"[Title/Abstract] OR "surgery"[Title/Abstract] OR "surgical"[Title/Abstract]
N=2,559,850
2. "Tray"[Title/Abstract] OR "pack"[Title/Abstract]
N=19.097
3. 1 and 2
N=2.109
4. Limited to December 28th, 2021, English language, Humans
N=1490
EMBASE
1. "Procedure:ti,ab,kw OR procedural:ti,ab,kw OR surgery:ti,ab,kw N=3482808
2. tray:ti,ab,kw OR pack:ti,ab,kw N=30719
3. 1 AND 2 N=4364
4. Limited to Articles, English language, excluding MEDLINE records
N=273
CINAHL
1. AB (Procedure or procedural or surgical) OR TI (Procedure or procedural or surgical) N=563200
2. AB (Tray or pack) OR TI (Tray or pack) N=7573
3. 1 and 2 N=866
4. 3 limited to December 28th, 2021, English language, excluded MEDLINE records N=308 Permalink https://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,sso&db=rzh&bquery=(+AB+(+Tray+ or+pack+)+OR+TI+ (+Tray+ or+pack+)+)+AND+(+AB+(+Procedure+or+procedural+or+surgery+or+surgical+)+OR+TI+(+Procedure+or+procedural+or+surgery+or+surgical+)+)&cli0=DT1&clv0=000001202112&cli1=LA1&clv1=Y&cli2=MX1& clv2=Y&cli3=LA99&clv3=eng&type=1&searchMode=Standard&site=ehost-live&scope=site
WEB OF SCIENCE
1. #1 AND #2 15,492 Add to query
2 TI=(tray or pack) OR AB=(tray or pack) OR KP=(tray or pack) 253,471 Add to query
1 TI = (Procedure or procedural or surgery or surgical) OR AB= (Procedure or procedural or surgery or surgical) OR KP=(Procedure or procedural or surgery or surgical)
3,261,025 #1 AND #2 and Surgery (Web of Science Categories) 9:52 AM Web of Science Core Collection Show editions 2.607
COCHRANE LIBRARY
1. #1 (Procedure or procedural or surgical):ti,ab,kw N=428,126
2. #2 (tray or pack):ti,ab,kw N=4240
3. #3 #1 and #2 n=1187
Last search: 12/22/2021 10:18
Appendix 2. Bibliography of selected studies on full text
Agarwal, R., N, Y. Sannappavar, Appukuttan, A., Ashok, A., &Rajanbabu, A. (2019). A prospective study evaluating
the impact of implementing ’bundled interventions’ in reducing surgical site infections among patients undergoing.
surgery for gynaecological Malignancies. Eur J ObstetGynecolReprod Biol, 243, 21-25. https://doi.org/10.1016/j.ejogrb. 2019.10.007
Akridge, J. (2004). Kits and trays: expanding on a value-driven product. Healthcare Purchasing News, 28(4), 16-20. Alfred, M., Catchpole, K., Hufer, E., Fredendall, L., & Taafe, K. M. (2021). Work systems analysis of sterile processing: assembly. BMJ Qual Saf, 30(4), 271-282. https://doi.org/10.1136/bmjqs-2019-010740
Arslan, F., &Yildiz, C. A. (2018). A Practical Suggestion for Prepare Dorsal Onlay Graft. J Craniofac Surg, 29(4), e344-e345. https://doi.org/10.1097/scs.0000000000004273
Baskett, T. F. (2004). Surgical management of severe obstetric hemorrhage: experience with an obstetric hemorrhage equipment tray. J ObstetGynaecol Can, 26(9), 805-808. https://doi.org/10.1016/s1701-2163(16)30152-9
Bhumisirikul, W., Bhumisirikul, P., &Pongchairerks, P. (2003). Long-term storage of small surgical instruments in autoclaved packages. Asian J Surg, 26(4), 202-204. https://doi.org/10.1016/s1015-9584(09)60303-1
Blanc, E., Bombail, M., Magnin, S., Paysant-Vallas, M., Falquet, B., Rochefort, F., &Corvaisier, S. (2017). Quality of sterilization process evaluated with two new indicators: Accuracy of the information transmitted and impact on the process of surgical tray composition. PharmacienHospitalier et Clinicien, 52(1), 64-70. https://doi.org/10.1016/j.phclin.2016.07.008
Bussières, M., L’Espèrance, S., Coulombe, M., &Rhainds, M. (2017). EVALUATION OF THE SURGICAL TRAY OPENING PROCEDURE IN OPERATING SUITES: SYSTEMATIC REVIEW AND RECOMMENDATIONS. Ornac j, 35(1), 57-66.
Byrnes, Jenifer N., Schmitt, Jennifer J., Tommaso, Christopher P., &Occhino, John A. (2017). Cost reduction techniques in the operating suite: Surgical tray optimization: 72. American Journal of Obstetrics and Gynecology, 216, S616.
Capra, R., Bini, S. A., Bowden, D. E., Etter, K., Callahan, M., Smith, R. T., & Vail, T. P. (2019). Implementing a perioperative efficiency initiative for orthopedic surgery instrumentation at an academic center: A comparative before-and-after study. Medicine (Baltimore), 98(7), e14338. https://doi.org/10.1097/md.0000000000014338
Chin, Christopher J., Sowerby, Leigh J., John-Baptiste, Ava, & Rotenberg, Brian W. (2014). Reducing otolaryngology surgical inefficiency via assessment of tray redundancy. Journal of Otolaryngology -- Head & Neck Surgery, 43(1), 46-49. https://doi.org/10.1186/s40463-014-0046-2
Cichos, K. H., Hyde, Z. B., Mabry, S. E., Ghanem, E. S., Brabston, E. W., Hayes, L. W., . . . Ponce, B. A. (2019). Optimization of Orthopedic Surgical Instrument Trays: Lean Principles to Reduce Fixed Operating Room Expenses. J Arthroplasty, 34(12), 2834-2840. https://doi.org/10.1016/j.arth.2019.07.040
Cichos, K. H., Linsky, P. L., Wei, B., Minnich, D. J., &Cer folio, R. J. (2017). Cost Savings of Standardization of Thoracic Surgical Instruments: The Process of Lean. Ann Thorac Surg, 104(6), 1889-1895. https://doi.org/10.1016/j.athoracsur. 2017.06.064
Costa, D. M., Lopes, L. K. O., Vickery, K., Watanabe, E., Vasconcelos, Lsnol, de Paula, M. C., . . . Tipple, A. F. V. (2018). Reprocessing safety issues associated with complex-design orthopaedic loaned surgical instruments and implants. Injury, 49(11), 2005-2012. https://doi.org/10.1016/j.injury.2018.09.006
Crosby, L., Lortie, E., Rotenberg, B., & Sowerby, L. (2020). Surgical Instrument Optimization to Reduce Instrument Processing and Operating Room Setup Time. Otolaryngol Head Neck Surg, 162(2), 215-219. https://doi.org/10.1177/ 0194599819885635
Dekonenko, C., Oyetunji, T. A., &Rentea, R. M. (2020). Surgical tray reduction for cost saving in pediatric surgical cases: A qualitative systematic review. J Pediatr Surg, 55(11), 2435-2441. https://doi.org/10.1016/j.jpeds urg.2020.05.010 Dieryck, K., Mertens, W., &Zeyen, T. (1998). Costsaving in the operating room: the Procedure Pack. Bull Soc BelgeOphtalmol, 270, 47-49.
Dilworth, J. P., & White, R. J. (1992). Postoperative chest infection after upper abdominal surgery: an important problem for smokers. Respir Med, 86(3), 205-210. https://doi.org/10.1016/s0954-6111(06)80056-9
Dobson, G., Seidmann, A., Tilson, V., &Froix, A. (2015). Confguring surgical instrument trays to reduce costs. IIE Transactions on Healthcare Systems Engineering, 5(4), 225-237. https://doi.org/10.1080/19488300.2015.1094759
Dos Santos, B. M., Fogliatto, F. S., Zani, C. M., & Peres, F. A. P. (2021). Approaches to the rationalization of surgical instrument trays: scoping review and research agenda. BMC Health Serv Res, 21(1), 163. https://doi.org/10.1186/s12913-021-06142-8
Dyas, A. R., Lovell, K. M., Balentine, C. J., Wang, T. N., Porterfeld, J. R., Jr., Chen, H., & Lindeman, B. M. (2018). Reducing cost and improving operating room efficiency: examination of surgical instrument processing. J Surg Res, 229, 15-19. https://doi.org/10.1016/j.jss. 2018.03.038
Edlich, R. F., Jones, K. C., Jr., Buchanan, L., Morgan, R. G., McGregor, W., &Himel, H. N. (1992). A disposable emergency wound treatment kit. J Emerg Med, 10(4), 463-467. https://doi.org/10.1016/0736-4679(92)90276-y
Egan, P., Pierce, A., Flynn, A., Teeling, S. P., Ward, M., & McNamara, M. (2021). Releasing operating room nursing time to care through the reduction of surgical case preparation time: A lean six sigma pilot study. International Journal of Environmental Research and Public Health, 18(22). https://doi.org/10.3390/ijerph182212098
Eggleston, M. K., Jr., Wax, J. R., Philput, C., Eggleston, M. H., & Weiss, M. I. (1997). Use of surgical pass trays to reduce intraoperative glove perforations. J Matern Fetal Med, 6(4), 245-247.
Fadaak, R., Davies, J. M., Blaak, M. J., Conly, J., Haslock, J., Kenny, A., . . . Leslie, M. (2021). Rapid conversion of an in-patient hospital unit to accommodate COVID-19: An interdisciplinary human factors, ethnography, and infection prevention and control approach. PLoS One, 16(1), e0245212. https://doi.org/10.1371/journ al.pone.0245212
Farrelly, J. S., Clemons, C., Witkins, S., Hall, W., Christison-Lagay, E. R., Ozgediz, D. E., . . . Caty, M. G. (2017). Surgical tray optimization as a simple means to decrease perioperative costs. Journal of Surgical Research, 220, 320-326. https:// doi.org/10.1016/j.jss. 2017.06.029
Farrokhi, F. R., Gunther, M., Williams, B., & Blackmore, C. C. (2015). Application of Lean Methodology for Improved Quality and Efciency in Operating Room Instrument Availability. J Healthc Qual, 37(5), 277-286. https://doi.org/10. 1111/jhq. 12053
Fogliatto, F. S., Anzanello, M. J., Tortorella, G. L., Schneider, D. S. S., Pereira, C. G. R., & Schaan, B. D. (2018). A Six Sigma Approach to Analyze Time-to-Assembly Variance of Surgical Trays in a Sterile Services Department. J Healthc Qual, 40(3), e46-e53. https://doi.org/10.1097/jhq. 0000000000000078
Glaser, B., Dänzer, S., &Neumuth, T. (2015). Intra-operative surgical instrument usage detection on a multi-sensor table. Int J Comput Assist Radiol Surg, 10(3), 351-362. https://doi.org/10.1007/s11548-014-1066-0
Grodsky, J. D., Theophanous, C. N., Schechet, S. A., Veldman, P. B., &Hariprasad, S. M. (2020). Reducing instruments in a vitrectomy surgical tray: Cost savings and results from a major academic hospital. International Journal of Retina and Vitreous, 6(1). https://doi.org/10.1186/s40942-020-00215-2
Halbert, R. J., Simon, R. R., &Leshuk, L. (1988). Logistics of medical care in rural Afghanistan. Ann Emerg Med, 17(8), 770-774. https://doi.org/10.1016/s0196-0644(88)80549-3
Harvey, L., Slocum, P., Heft, J., Van Meter, M., Lovett, B., & Adam, R. (2017). Gynecologic Surgery Instrument Trays: Leveraging Surgeon Knowledge to Improve Supply Chain Efciency. Journal of Gynecologic Surgery, 33(5), 180-183. https://doi.org/10.1089/gyn.2017.0039
Haya, N., Feiner, B., Baessler, K., Christmann-Schmid, C., & Maher, C. (2018). Perioperative interventions in pelvic organ prolapse surgery. Cochrane Database Syst Rev, 8(8), Cd013105. https://doi.org/10.1002/14651858.cd013105
Holdsworth, Jill. (2021). Targeting Zero—preventing Surgical Site Infections by Reducing Immediate Use Steam Sterilization (IUSS) of Surgical Instruments...Association for Professionals in Infection Control and Epidemiology, Annual Conference (Virtual), 28-30 June, 2021. American Journal of Infection Control, 49(6), S5-S5. https://doi.org/10.1016/j. ajic.2021.04.019
Huang, Y., Chen, Y., Pan, W., Hu, J., & Yi, L. (2021). Factors affecting implementation and pass rates of surgical instrument moistening. BMC Infect Dis, 21(1), 752. https://doi.org/10.1186/s12879-021-06471-3
Igesund, Unni, Overvåg, Grete, Rasmussen, Guri, &Rekvig, Ole Petter. (2019). Mapping of procedures for set-up of instruments in the sterile field for surgery. Norwegian Journal of Clinical Nursing / SykepleienForskning, 1-24. https:// doi.org/10.4220/Sykepleienf.2019.78413
John-Baptiste, A., Sowerby, L. J., Chin, C. J., Martin, J., & Rotenberg, B. W. (2016). Comparing surgical trays with redundant instruments with trays with reduced instruments: a cost analysis. CMAJ Open, 4(3), E404-e408. https://doi.org/ 10.9778/cmajo.20150092
Kaygusuz, I., Kizirgil, A., Karlidağ, T., Yalçin, S., Keles, E., Yakupoğullari, Y., &Alpay, C. (2003). Bacteriemia in septoplasty and septorhinoplasty surgery. Rhinology, 41(2), 76-79.
Khurana, S., Saini, S. S., Sundaram, V., Dutta, S., & Kumar, P. (2018). Reducing Healthcare-associated Infections in Neonates by Standardizing and Improving Compliance to Aseptic Non-touch Techniques: A Quality Improvement Approach. Indian Pediatr, 55(9), 748-752.
Kirmse, G., & Graf, M. (2021). Comparison of the cleaning and drying performance of different instrument tray designs and accessories. Zentralsterilisation - Central Service, 29(1), 42-47.
Knowles, M., Gay, S. S., Konchan, S. K., Mendes, R., Rath, S., Deshpande, V., . . . Wood, B. C. (2021). Data analysis of vascular surgery instrument trays yielded large cost and efciency savings. J Vasc Surg, 73(6), 2144-2153. https://doi.org/ 10.1016/j.jvs. 2020.09.043
Kong, K. C., Sheppard, M., &Serne, G. (1994). Dispensing surgical gloves onto the open surgical gown pack does not increase the bacterial contamination rate. J Hosp Infect, 26(4), 293-296. https://doi.org/10.1016/0195-6701(94)90020-5
Koyle, M. A., AlQarni, N., Odeh, R., Butt, H., Alkahtani, M. M., Konstant, L., . . . Baker, G. R. (2018). Reduction and standardization of surgical instruments in pediatric inguinal hernia repair. J PediatrUrol, 14(1), 20-24. https://doi.org/10. 1016/j.jpurol. 2017.08.002
Kusuda, K., Yamashita, K., Ohnishi, A., Tanaka, K., Komino, M., Honda, H., . . . Ohta, Y. (2016). Management of surgical instruments with radio frequency identification tags. Int J Health Care Qual Assur, 29(2), 236-247. https://doi.org/10. 1108/ijhcqa-03-2015-0034
Kwaan, M. R., Weight, C. J., Carda, S. J., Mills-Hokanson, A., Wood, E., Rivard-Hunt, C., &Argenta, P. A. (2016). Abdominal closure protocol in colorectal, gynecologic oncology, and urology procedures: a randomized quality improvement trial. Am J Surg, 211(6), 1077-1083. https://doi.org/10.1016/j.amjsurg.2015.10.032
Lin, D. M., Carson, K. A., Lubomski, L. H., Wick, E. C., & Pham, J. C. (2018). Statewide Collaborative to Reduce Surgical Site Infections: Results of the Hawaii Surgical Unit-Based Safety Program. J Am Coll Surg, 227(2), 189-197.e181. https:// doi.org/10.1016/j.jamco llsurg.2018.04.031
Litrico, Stéphane, Recanati, Geofrey, Gennari, Antoine, Maillot, Cédric, Safarini, Mo, &Huec, Jean-Charles. (2016). Single-use instrumentation in posterior lumbar fusion could decrease incidence of surgical site infection: a prospective bi-centric study. European Journal of Orthopaedic Surgery & Traumatology, 26(1), 21-26. https://doi.org/10.1007/ s00590-015-1692-4
Littell, J. J. (1951). New technique in the use of a surgical tray. AMA Arch Otolaryngol, 54(2), 195-197. Lonner, J. H., Goh, G. S., Sommer, K., Niggeman, G., Levicof, E. A., Vernace, J. V., & Good, R. P. (2021). Minimizing Surgical Instrument Burden Increases Operating Room Efficiency and Reduces Perioperative Costs in Total Joint Arthroplasty. J Arthroplasty, 36(6), 1857-1863. https://doi.org/10.1016/j.arth.2021.01.041
Malone, E., Baldwin, J., Richman, J., Lancaster, R., Krontiras, H., & Parker, C. (2019). The Impact of Breast Lumpectomy Tray Utilization on Cost Savings. J Surg Res, 233, 32-35. https://doi.org/10.1016/j.jss. 2018.06.063
Marchand, K. B., Taylor, K. B., Salem, H. S., Mont, M. A., & Marchand, R. C. (2020). Surgical Tray Optimization and Efficiency: The Impact of a Novel Sealed Sterile Container and Instrument Tray Technology. Surg Technol Int, 37, 349-355. Martinez, C., Omesiete, P., Pandit, V., Thompson, E., Nocera, M., Riall, T., . . . Nfonsam, V. (2020). A Protocol-Driven Reduction in Surgical Site Infections After Colon Surgery. J Surg Res, 246, 100-105. https://doi.org/10.1016/j.jss. 2019.08.018
McDermott, A. M., O’Cathain, E., Carey, B. W., O’Sullivan, P., & Sheahan, P. (2016). Sphenopalatine Artery Ligation for Epistaxis: Factors Infuencing Outcome and Impact of Timing of Surgery. Otolaryngol Head Neck Surg, 154(3), 547-552. https://doi.org/10.1177/0194599815620134
Meals, C. G., & Meals, R. A. (2007). A history of surgery in the instrument tray: eponymous tools used in hand surgery. J Hand SurgAm, 32(7), 942-953. https://doi.org/10.1016/j.jhsa. 2007.05.007
Moccia, G., Motta, O., Pironti, C., Proto, A., Capunzo, M., & De Caro, F. (2020). An alternative approach for the decontamination of hospital settings. J Infect Public Health, 13(12), 2038-2044. https://doi.org/10.1016/j.jiph. 2020.09.020
Nadeau, Kara. (2018). The quest for consistency: Product and process standardization drives effective kit/procedure tray planning. Healthcare Purchasing News, 42(11), 18-20.
Nast, K., & Swords, K. A. (2019). Decreasing operating room costs via reduction of surgical instruments. J PediatrUrol,15(2), 153.e151-153.e156. https://doi.org/10.1016/j.jpurol.2019.01.013
Navi, Fina, Motamedy, Mohammad HoseinKalantar, Faiaz, Fariba, Valayi, Naser, Lasemi, Eshagh, Hovakhti, Afagh, &Alavi, Amin. (2012). Action research for controlling bacterial contamination of surgery Sets sterilized by CSR war autoclave. Journal of Research in Dental Sciences, 9(2), 1p-1p.
Nguyen, J. M. V., Sadeghi, M., Gien, L. T., Covens, A., Kupets, R., Nathens, A. B., & Vicus, D. (2019). Impact of a preventive bundle to reduce surgical site infections in gynecologic oncology. Gynecol Oncol, 152(3), 480-485. https://doi.org/10. 1016/j.ygyno.2018.09.008
O’Donnell, P. (2002). Viewpoint. Taking control of custom procedure packs. SSM, 8(3), 16-18.
Osborn, L. K., & Hertz, E. (1999). Revising surgical instrument trays. Surgical Services Management, 5(6), 35-38.
Panahi, P., Stroh, M., Casper, D. S., Parvizi, J., & Austin, M. S. (2012). Operating room traffic is a major concern during total joint arthroplasty. Clin OrthopRelat Res, 470(10), 2690-2694. https://doi.org/10.1007/s11999-012-2252-4
Parker, P. J. (2006). (ii) Initial medical and surgical management. Current Orthopaedics, 20(5), 333-395.
Passey, A. (2002). Custom procedure trays in Europe: the strategic challenges. News Review (09637974)(138), 10-10.
Rao, M. K., Reilley, T. E., Schuller, D. E., & Young, D. C. (1992). Analysis of risk factors for postoperative pulmonary complications in head and neck surgery. Laryngoscope, 102(1), 45-47. https://doi.org/10.1288/00005537-199201000-00008
Reams, C. A. (2013). What’s on your surgical tray? Journal of the Dermatology Nurses’ Association, 5(6), 346-347. https:// doi.org/10.1097/JDN.0000000000000003
Schiavone, M. B., Moukarzel, L., Leong, K., Zhou, Q. C., Afonso, A. M., Iasonos, A., . . . Zivanovic, O. (2017). Surgical site infection reduction bundle in patients with gynecologic cancer undergoing colon surgery. Gynecol Oncol, 147(1), 115-119. https://doi.org/10.1016/j.ygyno.2017.07.010
Schwartz, J. L., Kirkpatrick, L., Hillebrecht, K. E., Lee, J. S., Steiman, J. G., Soran, A., Diego, E. J. (2021). Cutting Instruments to Cut Costs: A Simple Initiative with Breast Surgical Operating Room Trays that Resulted in Substantial Savings. Ann Surg Oncol, 28(10), 5553-5557. https://doi.org/10.1245/s10434-021-10496-y
Schömig, F., Perka, C., Pumberger, M., &Ascherl, R. (2020). Implant contamination as a cause of surgical site infection in spinal surgery: are single-use implants a reasonable solution? - a systematic review. BMC MusculoskeletDisord, 21(1),634. https://doi.org/10.1186/s12891-020-03653-z
Sebben, J. E. (1988). Sterile technique and the prevention of wound infection in office surgery--Part I. J Dermatol Surg Oncol, 14(12), 1364-1371. https://doi.org/10.1111/j.1524-4725.1988.tb01127.x
Sheth, S. S. (2003). Pneumo-surgical pack-a preliminary report. Int J GynaecolObstet, 81(2), 213-215. https://doi.org/ 10.1016/s0020-7292(03)00078-x
Shim, S. S., Danford, N. C., Wright, M. L., Abernathie, L. A. V., Kim, J. S., Kadiyala, R. K., &Vosseller, J. T. (2021). Economic Impact of Unused Surgical Instruments in an Orthopaedic Surgery Department at an Academic Medical Center: A Prospective Cross-sectional Study. J Surg Orthop Adv, 30(3), 131-135.
Siegel, G. W., Patel, N. N., Milshteyn, M. A., Buzas, D., Lombardo, D. J., &Morawa, L. G. (2015). Cost Analysis and Surgical Site Infection Rates in Total Knee Arthroplasty Comparing Traditional vs. Single-Use Instrumentation. J Arthroplasty, 30(12), 2271-2274. https://doi.org/10.1016/j.arth.2015.05.037
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. https://doi.org/10.1136/bmj.l4898. PMID: 31462531.
Stockert, E. W., &Langerman, A. (2014). Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. Journal of the American College of Surgeons, 219(4), 646-655. https://doi.org/10.1016/j. jamcollsurg.2014.06.019
Strulak, L., Gronki, F., Shariat, K., Schöni, D., & Alferi, A. (2021). Eponyms of Cranial Neurosurgical Instruments: An International Collaboration to Optimize the Field of Neurosurgery. World Neurosurg, 153, 26-35. https://doi.org/10. 1016/j.wneu.2021.06.073
Strzelecki, L. R., & Nelson, J. H. (1989). Evaluation of closed container flash sterilization system. OrthopNurs, 8(1), 21-24. https://doi.org/10.1097/00006416-198901000-00006
Thiel, C. L., Fiorin Carvalho, R., Hess, L., Tighe, J., Laurence, V., Bilec, M. M., &Baratz, M. (2019). Minimal Custom Pack Design and Wide-Awake Hand Surgery: Reducing Waste and Spending in the Orthopedic Operating Room. Hand (NY), 14(2), 271-276. https://doi.org/10.1177/1558944717743595
Torres, A., Inzunza, M., Jarry, C., Serrano, F., Varas, J., & Zavala, A. (2021). DEVELOPMENT AND VALIDATION OF A NEW LAPAROSCOPIC ENDOTRAINER FOR NEONATAL SURGERY AND REDUCED SPACES. Arq Bras Cir Dig, 33(4), e1559. https:// doi.org/10.1590/0102-672020200004e1559
Tseng, J., Sax, H. C., Gewertz, B. L., Margulies, D. R., & Alban, R. F. (2020). Surgical Supply Cost Awareness Is Associated With Lower Costs: A Single-Center Experience. Am Surg, 86(10), 1407-1410. https://doi.org/10.1177/0003134820964494
Van Meter, M. M., & Adam, R. A. (2016). Costs associated with instrument sterilization in gynecologic surgery. Am J ObstetGynecol, 215(5), 652.e651-652.e655. https://doi.org/10.1016/j.ajog.2016.06.019
Wagner, J. A., Dexter, F., Greeley, D. G., & Schreiber, K. (2021). Operating room air delivery design to protect patient and surgical site results in particles released at surgical table having greater concentration along walls of the room than at the instrument tray. Am J Infect Control, 49(5), 593-596. https://doi.org/10.1016/j.ajic.2020.10.003
Wannemuehler, T. J., Elghouche, A. N., Kokoska, M. S., Deig, C. R., & Matt, B. H. (2015). Impact of Lean on surgical instrument reduction: Less is more. Laryngoscope, 125(12), 2810-2815. https://doi.org/10.1002/lary.25407
Watters, T. S., Mather, R. C., 3rd, Browne, J. A., Berend, K. R., Lombardi, A. V., Jr., &Bolognesi, M. P. (2011). Analysis of procedure-related costs and proposed benefits of using patient-specific approach in total knee arthroplasty. J Surg Orthop Adv, 20(2), 112-116.
Weber, L. A. (1998). The surgical tray. Dermatol Clin, 16(1), 17-24. https://doi.org/10.1016/s0733-8635(05)70484-8 Weiser, M. C., Shemesh, S., Chen, D. D., Bronson, M. J., &Moucha, C. S. (2018). The Efect of Door Opening on Positive Pressure and Airflow in Operating Rooms. J Am AcadOrthop Surg, 26(5), e105-e113. https://doi.org/10.5435/jaaos-d- 16-00891
Wells, Rick. (2017). Can using custom trays reduce instrument repairs? Healthcare Purchasing News, 41(6), 78-79.
Wells, Rick. (2018). Hospital improves costs, efficiencies with custom trays: Part 2: Study completion delivers impressive results. Healthcare Purchasing News, 42(5), 60-61.
Wood, B. C., Konchan, S., Gay, S., Rath, S., Deshpande, V., & Knowles, M. (2021). Data Analysis of Plastic Surgery Instrument Trays Yields Significant Cost Savings and Efficiency Gains. Ann Plast Surg, 86(6S Suppl 5), S635-s639. https://doi. org/10.1097/sap.0000000000002913
Appendix 2.1: Excluded articles with cause
– Full text non disponibile (9): Littell 1951, Sebben 1988, Sheth 2003, Dieryck 1998, Stephanie 2010, Akridge 2004, Osborne 1999, Passey 2002, Wilkie 1986
– Outcome non descritti o non d’interesse: (24) Ahmadi, 2019; Egan 2021, Baskett 2004, Bhumisorikul 2004, Costa 2018, Fogliatto 2018, Glaser 2015, Halbert 1988, Huang 2021, Kimse 2021, Kusuda 2016, Strulak 2021; Strzelecki, 1989, Torres 2021, Bradley 2019, Alfred 2021, Dana Barlow 2015, Holdsworth 2021, Igesund 2019, Navi 2012, Parker 2006, Wells 2017, Wells 2018, Eggleston 1997
– Non focus su set chirurgici: (24) Agarwal 2019, Arslan 2018, Dilworth 1992, Edilich 1992, Fadaak 2021, Haya 2018, Johnson 2016, Kaygusuz 2003, Khurana 2018, Kong 1994; Kwaan 2016; Lin 2018, Martinez 2020; McDermott 2016; Meals 2007; Moccia 2020; Nguyen 2019; Panahi 2012; Rao 1992, Wagner 2021, Weiser 2018; Watters, 2011; Makram 2021, Olivere 2021
– – Study design not contemplated (es. editoriali, case reports, opinion papers) (6): Reams 2013, Weber 1998, Nadeau 2018, O’Donnell 2002, Stephanie 2010, Goldberg 2019
– Full text in altra lingua (1): Blanc 2017
References
1. Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, Tsai TC, Ko CY, Bilimoria KY. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313(5):483–95. https://doi.org/10.1001/jama.2014.18614.
2. Anderson DJ, Kaye KS, Chen LF, Schmader KE, Choi Y, Sloane R, Sexton DJ. Clinical and financial outcomes due to methicillin-resistant Staphylococcus aureus surgical site infection: a multi-center matched outcomes study. PLoS ONE. 2009;4(12):e8305. https://doi.org/10. 1371/journ al.pone.0008305.
3. Panahi P, Stroh M, Casper DS, Parvizi J, Austin MS. Operating room traffic is a major concern during total joint arthroplasty. Clin OrthopRelat Res. 2012;470(10):2690–4. https://doi.org/10.1007/s11999-012-2252-4.
4. Wagner JA, Dexter F, Greeley DG, Schreiber K. Operating room air delivery design to protect patient and surgical site results in particles released at surgical table having greater concentration along walls of the room than at the instrument tray. Am J Infect Control. 2021;49(5):593–6. https://doi.org/10.1016/j.ajic.2020.10.003.
5. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hofmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
6. Dos Santos BM, Fogliatto FS, Zani CM, Peres FAP. Approaches to the rationalization of surgical instrument trays: scoping review and research agenda. BMC Health Serv Res. 2021;21(1):163.
7. Schömig F, Perka C, Pumberger M, Ascherl R. Implant contamination as a cause of surgical site infection in spinal surgery: are single-use implants a reasonable solution? A systematic review. BMC MusculoskeletDisord. 2020;21(1):634.
8. Bussières M, L’Espèrance S, Coulombe M, Rhainds M. Evaluation of the surgical tray opening procedure in operating suites: systematic review and recommendations. ORNAC J. 2017;35(1):57–66.
9. Dekonenko C, Oyetunji TA, Rentea RM. Surgical tray reduction for cost saving in paediatric surgical cases: a qualitative systematic review. J PediatrSurg. 2020;55(11):2435–41.
10. Schiavone MB, Moukarzel L, Leong K, Zhou QC, Afonso AM, Iasonos A, Roche KL, Leitao MM Jr, Chi DS, Abu-Rustum NR, Zivanovic O. Surgical site infection reduction bundle in patients with gynecologic cancer undergoing colon surgery. Gynecol Oncol. 2017;147(1):115–9.
11. Litrico S, Recanati G, Gennari A, Maillot C, Safarini M, Huec J-C. Single-use instrumentation in posterior lumbar fusion could decrease incidence of surgical site infection: a prospective bi-centric study. Eur J Orthop Surg Traumatol. 2016;26(1):21–6.
12. Siegel GW, Patel NN, Milshteyn MA, Buzas D, Lombardo DJ, Morawa LG. Cost analysis and surgical site infection rates in total knee arthroplasty comparing traditional vs. single-use instrumentation. J Arthroplasty. 2015;30(12):2271–4.
13. Toor J, Du JT, Koyle M, Abbas A, Shah A, Bassi G, Morra D, Wolfstadt J. Inventory optimization in the perioperative care department using Kotter’s change model. Jt Comm J Qual Patient Saf. 2022;48(1):5–11.
14. Stockert EW, Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg. 2014;219(4):646–55.
15. Cichos KH, Linsky PL, Wei B, Minnich DJ, Cerfolio RJ. Cost savings of standardization of thoracic surgical instruments: the process of lean. Ann ThoracSurg. 2017;104(6):1889–95.
16. Dyas AR, Lovell KM, Balentine CJ, Wang TN, Porterfeld JR Jr, Chen H, Lindeman BM. Reducing cost and improving operating room efficiency: examination of surgical instrument processing. J Surg Res. 2018;229:15–9.
17. Thiel CL, Fiorin Carvalho R, Hess L, Tighe J, Laurence V, Bilec MM, Baratz M. Minimal custom pack design and wide-awake hand surgery: reducing waste and spending in the orthopedic operating room. Hand (N Y). 2019;14(2):271–6.
18. Byrnes JN, Schmitt JJ, Tommaso CP, Occhino JA. Cost reduction techniques in the operating suite: surgical tray optimisation: 72. Am J Obstet Gynecol. 2017;216:S616.
19. Capra R, Bini SA, Bowden DE, Etter K, Callahan M, Smith RT, Vail TP. Implementing a peri-operative efficiency initiative for orthopedic surgery instrumentation at an academic centre: a comparative before-and-after study. Medicine (Baltimore). 2019;98(7): e14338.
20. Crosby L, Lortie E, Rotenberg B, Sowerby L. Surgical instrument optimization to reduce instrument processing and operating room setup
time. Otolaryngol Head Neck Surg. 2020;162(2):215–9.
21. Cichos KH, Hyde ZB, Mabry SE, Ghanem ES, Brabston EW, Hayes LW, McGwin G Jr, Ponce BA. Optimization of orthopedic surgical instrument trays: lean principles to reduce fxed operating room expenses. J Arthroplasty. 2019;34(12):2834–40.
22. Chin CJ, Sowerby LJ, John-Baptiste A, Rotenberg BW. Reducing otolaryngology surgical inefficiency via assessment of tray redundancy. J Otolaryngol Head Neck Surg. 2014;43(1):46–9.
23. Dobson G, Seidmann A, Tilson V, Froix A. Confguring surgical instrument trays to reduce costs. IIE Trans Healthc Syst Eng. 2015;5(4):225–37.
24. Farrelly JS, Clemons C, Witkins S, Hall W, Christison-Lagay ER, Ozgediz DE, Cowles RA, Stitelman DH, Caty MG. Surgical tray optimization as a simple means to decrease perioperative costs. J Surg Res. 2017;220:320–6.
25. Farrokhi FR, Gunther M, Williams B, Blackmore CC. Application of lean methodology for improved quality and efficiency in operating room instrument availability. J Healthc Qual. 2015;37(5):277–86.
26. Fu TS, Msallak H, Namavarian A, Chiodo A, Elmasri W, Hubbard B, Xu J, Pegoraro R, Higgins K, Enepekides D, Monteiro E, Eskander A. Surgical tray optimization: a quality improvement initiative that reduces operating room costs. J Med Syst. 2021;45(8):78.
27. Grodsky JD, Theophanous CN, Schechet SA, Veldman PB, Hariprasad SM. Reducing instruments in a vitrectomy surgical tray: Cost savings and results from a major academic hospital. Int J Retin Vitr. 2020. https://doi.org/10.1186/s40942-020-00215-2.
28. Harvey L, Slocum P, Heft J, Van Meter M, Lovett B, Adam R. Gynecologic surgery instrument trays: leveraging surgeon knowledge to improve supply chain efciency. J Gynecol Surg. 2017;33(5):180–3.
29. John-Baptiste A, Sowerby LJ, Chin CJ, Martin J, Rotenberg BW. Comparing surgical trays with redundant instruments with trays with reduced instruments: a cost analysis. CMAJ Open. 2016;4(3):E404-e408.
30. Knowles M, Gay SS, Konchan SK, Mendes R, Rath S, Deshpande V, Farber MA, Wood BC. Data analysis of vascular surgery instrument trays yielded large cost and efficiency savings. J VascSurg. 2021;73(6):2144–53.
31. Koyle MA, AlQarni N, Odeh R, Butt H, Alkahtani MM, Konstant L, Pendergast L, Koyle LC, Baker GR. Reduction and standardization of surgical instruments in pediatric inguinal hernia repair. J PediatrUrol. 2018;14(1):20–4.
32. Lonner JH, Goh GS, Sommer K, Niggeman G, Levicof EA, Vernace JV, Good RP. Minimizing surgical instrument burden increases operating room efficiency and reduces perioperative costs in total joint arthroplasty. J Arthroplasty. 2021;36(6):1857–63.
33. Malone E, Baldwin J, Richman J, Lancaster R, Krontiras H, Parker C. The impact of breast lumpectomy tray utilization on cost savings. J Surg Res. 2019;233:32–5.
34. Marchand KB, Taylor KB, Salem HS, Mont MA, Marchand RC. Surgical tray optimization and efficiency: the impact of a novel sealed sterile container and instrument tray technology. SurgTechnol Int. 2020;37:349–55.
35. Nast K, Swords KA. Decreasing operating room costs via reduction of surgical instruments. J PediatrUrol. 2019;15(2):153.e1-153.e6.
36. Schwartz JL, Kirkpatrick L, Hillebrecht KE, Lee JS, Steiman JG, Soran A, Johnson RR, McAulife PF, Diego EJ. Cutting instruments to cut costs: a simple initiative with breast surgical operating room trays that resulted in substantial savings. Ann Surg Oncol. 2021;28(10):5553–7.
37. Shim SS, Danford NC, Wright ML, Abernathie LAV, Kim JS, Kadiyala RK, Vosseller JT. Economic impact of unused surgical instruments in an orthopaedic surgery department at an academic medical center: a prospective cross-sectional study. J SurgOrthop Adv. 2021;30(3):131–5.
38. Tseng J, Sax HC, Gewertz BL, Margulies DR, Alban RF. Surgical supply cost awareness is associated with lower costs: a single-centre experience. Ann Surg Oncol. 2020;86(10):1407–10.
39. Van Meter MM, Adam RA. Costs associated with instrument sterilization in gynecologic surgery. Am J ObstetGynecol. 2016;215(5):652. e1-652.e5.
40. Wannemuehler TJ, Elghouche AN, Kokoska MS, Deig CR, Matt BH. Impact of Lean on surgical instrument reduction: Less is more. Laryngoscope. 2015;125(12):2810–5.
41. Wood BC, Konchan S, Gay S, Rath S, Deshpande V, Knowles M. Data analysis of plastic surgery instrument trays yields significant cost savings and efficiency gains. Ann Plast Surg. 2021;86:S635-s639.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the Discover Health Systems by Wound World.


