Introduction
Surgical site infections (SSI) are a major concern after inguinal vascular surgery, increasing inpatient stay and patient morbidity [1]. It has also been reported that approximately 55% of all SSIs are preventable with the implementation of the current evidence-based strategies [2]. Decreasing SSI incidence is therefore highly prioritized.
Use of negative pressure wound therapy (NPWT), dressings with an active suction, on sutured incisions has for the last decade been proposed as a possible prevention of SSIs. The evidence on its efficacy has increased in recent years, with five meta-analyses of randomized controlled trials (RCTs) showing significant reductions in SSI incidence when using NPWT compared to standard dressings [3–7]. However, the included studies evaluated SSI incidence after mainly open inguinal revascularization procedures. The risk of developing an SSI differs greatly between different inguinal vascular surgical procedures, with incidences varying between approximately 2–5% in inguinal incisions after endovascular aneurysm repair (EVAR) [1, 8, 9] and 20–30% after open revascularization procedures [3]. To date, no study has evaluated NPWT dressings on incisions after EVAR procedures only. The aim of the present RCT was to evaluate if NPWT dressings on inguinal incisions after EVAR procedures decrease the risk of developing an SSI.
Materials and methods
INVIPS-trial
The INVIPS-trial was approved by the regional ethical review board in Lund (Diary number 2013/322 and 2016/886). A study protocol was registered a priori at clinicaltrials.gov (NCT01913132) [8]. The INVIPS-trial was reported in accordance with the CONSORT guidelines (CONSORT checklist in Supplemental Table 1).
Population
All patients undergoing elective EVAR procedures between November 2013 and December 2020 at two vascular centers in Sweden, Ska ˚ne university hospital in Malmo¨ and O ¨ rebro university hospital, were considered for study participation. Preoperative exclusion criteria were inability to understand the study instructions and purpose, age \ 18 years old, inability to give informed consent or ongoing inguinal infection. Postoperative exclusion criteria were no inguinal incision (vascular hemostasis with percutaneous closure device [PCD] or non-inguinal arterial access); incorrect allocation of interventional or control wound dressings; withdrawn consent; re-operation with an inguinal incision for acute bleeding, peripheral ischemia, or stent graft-reintervention; or non-incisional related mortality within 90 days postoperatively. [8]
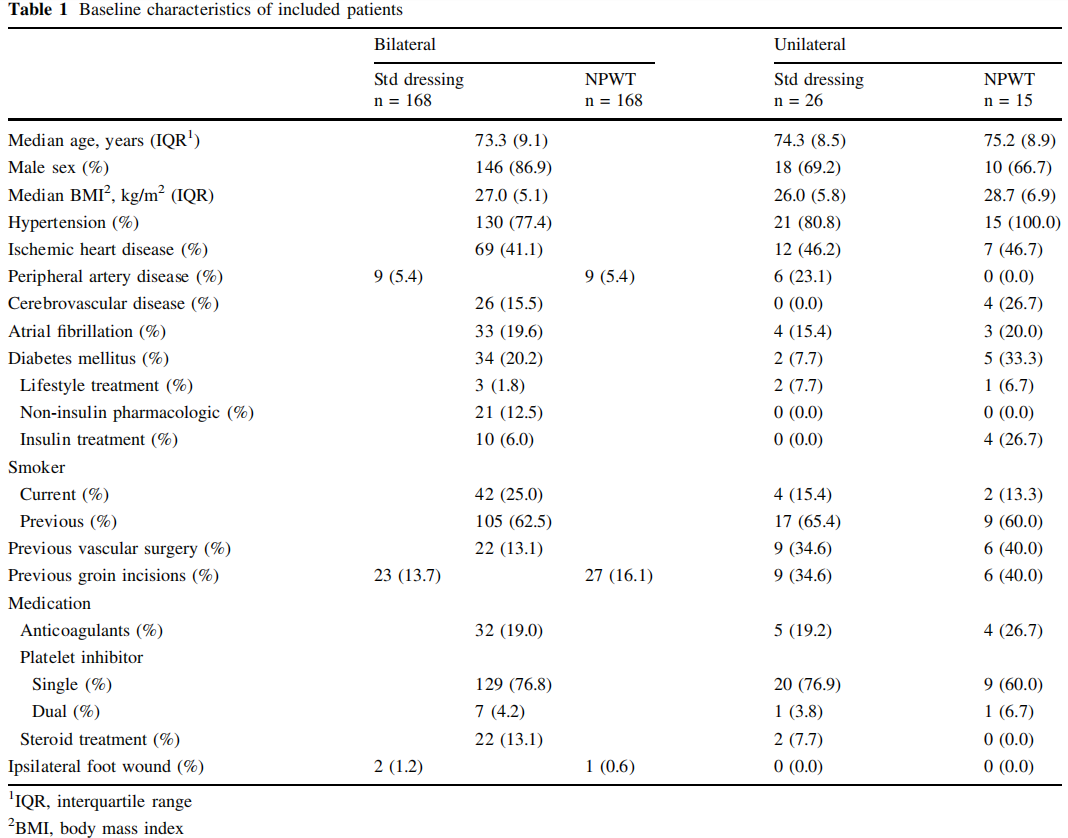
All types of elective EVAR procedures including fenestrated EVAR and thoracic EVAR were included. The preoperative antibiotic prophylaxes used was the combination of 800 mg sulfamethoxazole and 160 mg trimethoprim orally, cloxacillin 2 g intravenously or clindamycin 600 mg intravenously in case of penicillin allergy. The preoperative skin-preparation, constituted of preoperative shower, hair removal with electric clipper if necessary, and antiseptic skin preparation using chlorhexidine gluconate. The surgical incisions in EVAR procedures were performed at the end of the endovascular treatment to achieve local hemostasis at the arterial access site. The primary technique for arterial access closure was bilateral fascia closure (Fig. 1) [10, 11]. As a bale-out procedure, if hemostasis was not achieved by fascia closure, cut-down with exposure of the common femoral artery for direct arterial suturing (abbreviated cut-down) was performed. Occasionally, the artery was exposed further for patch angioplasty where a patch of autologous, xenogenous or synthetic material was sutured over the arterial wall deficit.
Eligible patients were given written information about the study per ordinary mail and oral information at the outpatient clinic before written informed consent was retrieved from participating patients. Informed consent was retrieved before the surgical procedure was conducted.
Randomization and wound dressing allocation
The enrolment of patients and the randomization process were conducted by nurses at the outpatient clinic, neither connected to the study investigators nor the operating surgeons. The random allocation sequence procedure was managed independently at the outpatient clinic by the draw of a randomization form from an opaque envelope prior to surgery. The randomization ratio was 1:1. Participating patients were randomized to receive either the NPWT dressing or the institutions’ standard dressing. In patients with bilateral incisions, the right inguinal incision was randomized while the contralateral incision received the opposite type of dressing. The NPWT dressing used was the PICOTM (Smith & Nephew, London, UK). The standard dressings used at the two centers during the sevenyear study period were: ViTri Pad (ViTri medical, Stockholm, Sweden), Tegaderm ? pad (3 M, Maplewood, Minnesota, US), Opsite post-op (Smith & Nephew, Hull, UK), or Mepilex boarder (Mo ¨lnlycke health care, Gothenburg, Sweden). Both the NPWT and standard dressings were applied under sterile conditions by the theater nurse while the patient was still at the operating theater. The NPWT dressings were changed if fully saturated with fluids or removed at 7 days postoperatively. The standard dressings were also changed if fully saturated with fluids or at the day of discharge.
Outcomes
The primary endpoint of the present RCT was SSI incidence at 90 days postoperatively [8]. The secondary endpoints were SSI incidence at one year postoperatively and other wound complications (hematoma, wound dehiscence and seroma or lymphatic complications) at 90 days and one year postoperatively.
SSIs were defined using the predefined ASEPSIS-score [12] and criteria issued by Centers for Disease Control (CDC) [13] (Supplemental Tables 2 and 3). Identified SSIs were also graded according to the Szilagyi classification: grade 1 (involving dermis), grade 2 (involving subcutaneous tissue), or grade 3 (involving vascular prosthesis) [14]. The wound complications, both SSIs and other wound complications, were also graded according to an incisional wound complication scale [15].
The clinical wound manifestations were assessed by nurses and physicians independent from the conducted study during inpatient care and at the vascular outpatient clinic with a standardized follow-up visit 30 days postoperatively. A phone interview at 90 days postoperatively was conducted by the study nurse. Data from visits at primary care, emergency room and other surgical or vascular centers were collected retrospectively. Nurses and physicians were blinded to wound dressing allocations apart from during postoperative inpatient care.
Adverse events related to the NPWT dressing such as pain, skin blisters or technical problems were sought for and registered throughout the trial.
Sample size
A sample size calculation was conducted a priori and has previously been published [8]. In the sample size calculation, the following assumptions were made: a reduction of SSI incidence through the use of NPWT dressing from 4.4 to 1%, a distribution of 80% bi and 20% unilateral incisions, a mortality rate at 1 year of 6.7%, an unspecified loss to follow-up of 10%, a statistical power of 80% and a significance level of 5%, resulting in an estimated demand of 497 incisions (398 bilateral and 99 unilateral). The enrolment of patients was terminated when the total number of randomized incisions was reached.
Statistics
Data management and statistical analyses were conducted using SPSS software version 27 for Windows (IBM Corporation, New York, USA). The primary and secondary outcomes were analyzed on an intention-to-treat basis and presented per incision. The primary outcome was presented as frequencies and odds ratios (OR) with 95% confidence intervals (CI). Uni and bilateral incisions were analyzed separately. For frequencies of descriptive data, Fisher’s exact test was used in unilateral incisions while McNemar’s test was used in bilateral incisions. The obtained pvalues of the separate analyses for unilateral and bilateral incisions were then combined using Fisher’s method of combining p-values [16]. P-values of \ 0.05 were considered significant.
Results
Patients
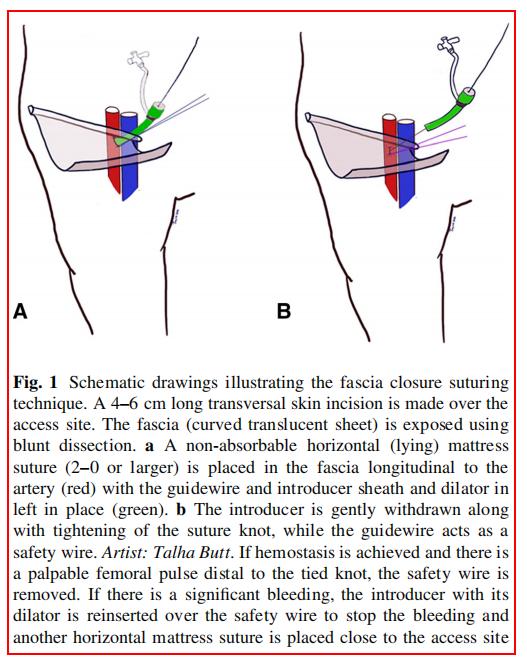
Four hundred and ninety patients were assessed for eligibility of which 275 patients were randomized and received inguinal incisions (223 bilaterally and 52 unilaterally), resulting in 498 incisions. Of these, 66 patients (121 incisions) were excluded: 45 patients (85 incisions) due to not receiving the randomized wound dressing and 21 patients (36 incisions) were lost to follow-up. In total, 336 bilateral and 41 unilateral incisions were finally included in the analysis of the primary endpoint (Fig. 2).
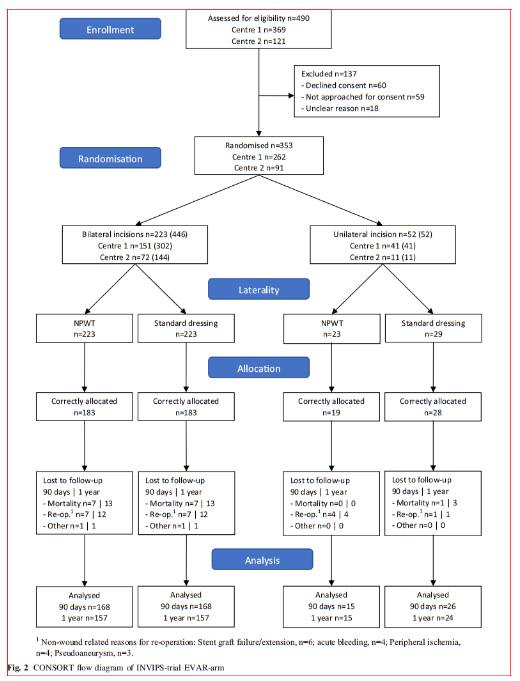
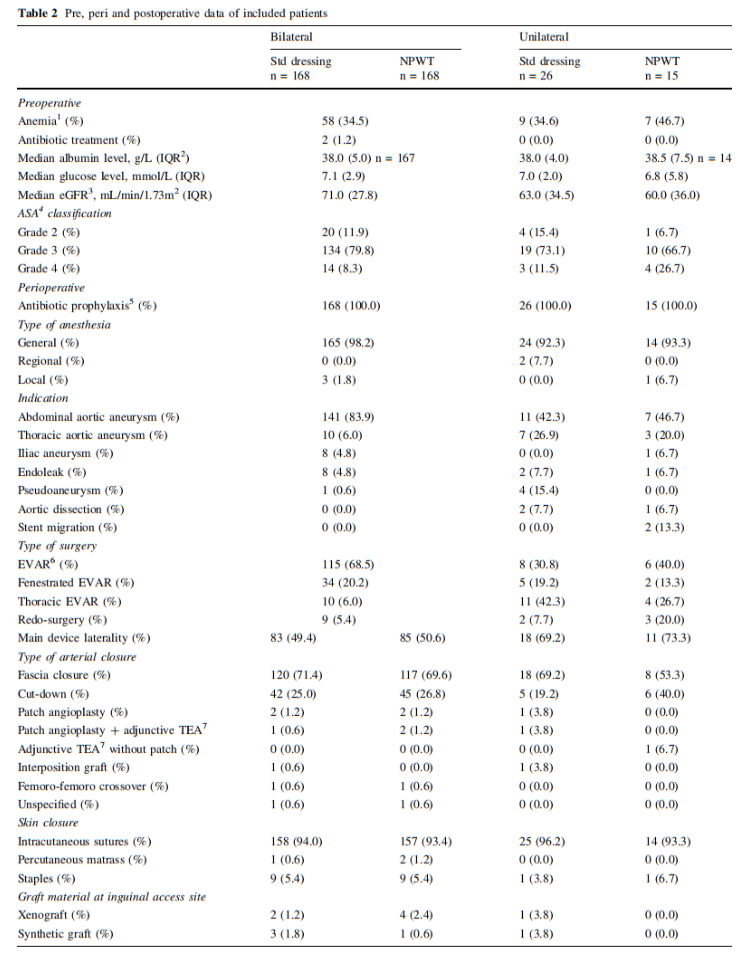
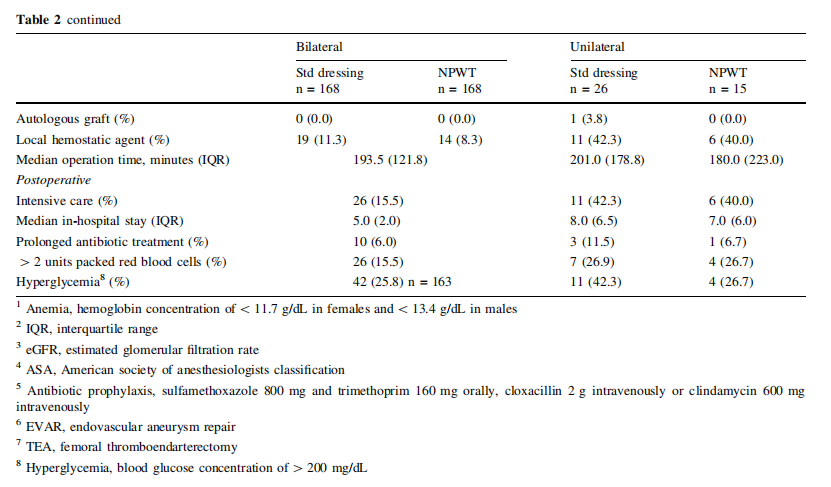
The patient characteristics are listed in Table 1 and pre, peri and postoperative parameters are listed in Table 2. In patients operated bilaterally, the patient-related characteristics and parameters are identical and no differences among limb associated parameters were detected. In the unilaterally operated incisions, there was no difference in patient or pre, peri and postoperative parameters.
Outcomes
The primary outcome, SSI incidence at 90 days postoperatively, was in patients operated bilaterally 1.8% (n = 3/ 168 [Center 1, n = 3/111; Center 2, n = 0/57]) in the NPWT and 4.8% (n = 8/168 [Center 1, n = 6/111; Center 2, n = 2/57]) in the standard dressing group (p = 0.18; OR 0.29 [95% CI 0.03–1.50]) and in patients operated unilaterally 13.3% (n = 2/15 [Center 1, n = 0/9; Center 2, n = 2/ 6]) in the NPWT and 11.5% (n = 3/26 [Center 1, n = 2/24; Center 2, n = 1/2]) in the standard dressing group (p = 1.0; OR 1.02 [95% CI 0.80–1.30]), using both the ASEPSIS score or the CDC criteria to define SSIs (combined p-value of 0.49) (Table 3).
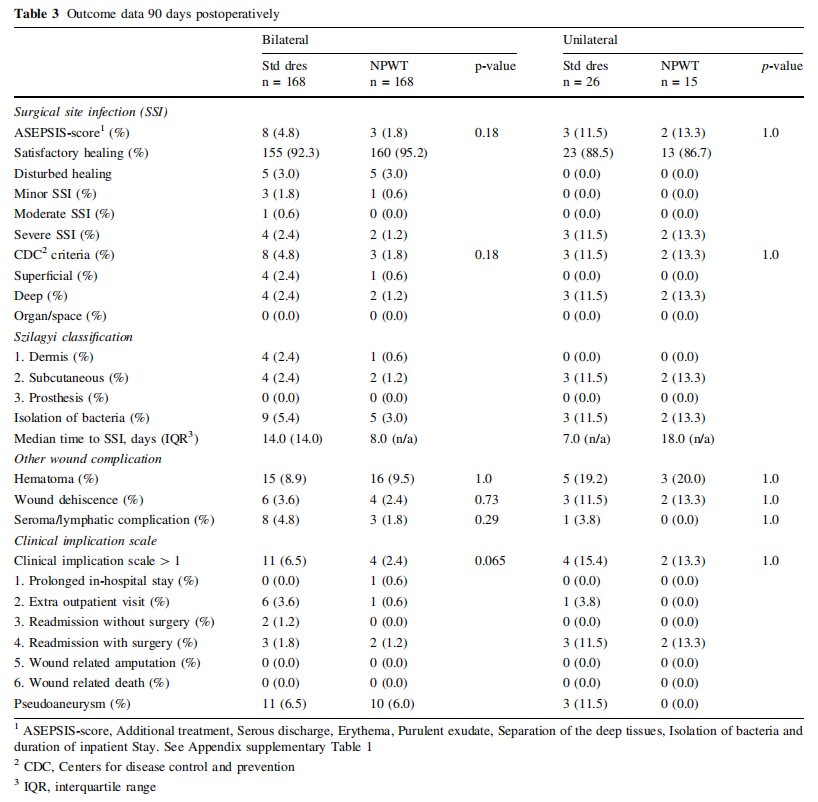
The secondary outcome, SSI incidence at one year postoperatively, was in patients operated bilaterally 1.9% (n = 3/157 [Center 1, n = 3/104; Center 2, n = 0/53]) in the NPWT and 4.5% (n = 7/157 [Center 1, n = 5/104; Center 2, n = 2/53]) in the standard dressing group (p = 0.29) and in patients operated unilaterally 13.3% (n = 2/15 [Center 1, n = 0/9; Center 2, n = 2/6]) in the NPWT and 12.5% (n = 3/24 [Center 1, n = 2/22; Center 2, n = 1/2]) in the standard dressing group (p = 1.0), using both the ASEPSIS score or the CDC criteria to define SSIs (combined p-value of 0.65).
All SSIs occurred within 90 days postoperatively, with no SSI diagnosed after 30 days (range 4–23 days). Most SSIs were diagnosed after hospital discharge, n = 10/16, 62,5% (bilateral: NPWT n = 1/3 [33.3%], Standard dressing n = 5/8 [62,5%]; unilateral: NPWT n = 2/2 [100.0%], Standard dressing n = 2/3 [66.7%]). In all SSIs, bacteria were isolated in microbiological analysis of incisional wound cultures (Supplemental Table 4). Sepsis was not reported following an SSI. One patient with bilateral incisions developed an aortic stent graft infection approximately 5 months postoperatively verified by PET-CT and microbiological culture from the aneurysm sac (Enterococcus faecalis). Both inguinal incisions healed without complications within 30 days postoperatively and the source of the aortic stent graft infection was never determined.
The other secondary outcomes, incidence of non-infectious wound complications (hematoma, wound dehiscence and seroma or lymphatic complications) showed no differences among incisions treated with NPWT compared to standard dressings (Table 3).
In bilaterally operated patients, 2.4% (n = 4/168) of the NPWT treated incisions and 6.5% (n = 11/168) of the standard dressing treated incisions (p = 0.065) received additional treatment according to the incisional wound complication scale. In unilaterally operated patients, 13.3% (n = 2/15) of the NPWT treated incisions and 15.4% (n = 4/26) of the standard dressing treated incisions (p = 1.0) needed additional treatment. No patient was amputated or died due to any incisional wound
Adverse events
Twelve of 183 (6.6%) patients receiving the NPWT reported adverse events. Nine did not tolerate the unit and tube (of which six had postoperative confusion), two experienced pain or discomfort and one was disturbed by the noise from the pump. Nine patients (5.4%) reported technical problems with the NPWT dressing, of which eight were leakage and one lack of suction. In eleven of 183 patients (6.0%), the NPWT treatment was discontinued prior to the recommended 7 days of treatment.
Discussion
The present multicenter RCT showed no evidence of difference in SSI incidence in these low-risk inguinal incisions when comparing NPWT with standard dressings after EVAR with the primary intent of fascia closure. This finding does not support the routine use of NPWT dressings in uncomplicated inguinal incisions with fascia closure.
There was a trend toward fewer additional treatments due to any incisional wound complication in bilateral incisions treated with NPWT compared to standard dressings, despite no significant difference in incidence of SSI or other incisional wound complications. This highlights the importance to also report the clinical implication of incisional wound complications.
The number of incisions randomized (n = 498) met the predefined number published in the study protocol (n = 497). The proportion of bilateral incisions (89.6%) were higher than anticipated based on the study protocol (80.0%), increasing the statistical power. Despite the high number of incisions excluded due to incorrect allocation of wound dressings, the bilateral incisions included in analysis (n = 336) almost met the predefined number for bilateral incisions only (n = 340). The number of included unilateral incisions was lower than anticipated based on the power calculation, which is due to a lower proportion of patients operated unilaterally and a higher fraction of incorrectly allocated wound dressings. Nevertheless, combining the number of bilateral and unilateral incisions resulted in an adequately powered study.
The present multicenter RCT is the first to evaluate NPWT dressings on closed incisions after EVAR procedures only. Previous RCTs have not separated EVAR incisions from other inguinal vascular incisions, resulting in a much higher SSI incidence due to a higher SSI incidence in open inguinal revascularization procedures. The SSI incidence in the present RCT is considered similar to that of previous studies evaluating SSI incidence in incisions after EVAR procedures only [1, 8, 9]. This confirms the importance of separating incisions in EVAR procedures from open revascularization procedures when evaluating interventions to reduce SSI incidence.
Despite monitoring the patients for one year to capture low virulent prosthetic SSI (according to the CDC guidelines) [12], none were detected. One patient developed an aortic stent graft infection, however of unknown origin. The incidence of aortic stent graft infections in the present study was lower than the incidence of 1.4% previously reported in a retrospective study [17]. The low incidence of aortic stent graft infections could be due to a too short follow-up time since the reported median time to aortic stent graft infection in that study was 3.2 years. [17] All the SSIs in the present study were diagnosed within 30 days postoperatively, indicating no need for a prolonged wound surveillance time of up to 90 days.
In recent years the use of percutaneous closure devices (PCDs) instead of fascia closure or cut-down has increased. One systematic review with meta-analysis of observational studies has shown a significant decrease in SSI and seroma incidence but an increase in pseudoaneurysm incidence with PCD compared to cut-down technique [18]. In two systematic reviews with meta-analyses of RCTs only, no significant difference in SSI incidence was seen [19, 20]. In one of the reviews [20], a significant decrease in seroma/lymphorrhea incidence with PCD was demonstrated. Only one RCT has evaluated PCD compared to fascia closure, showing no difference in incidence of SSI and other incisional wound complications [11]. The exact extent of contemporary use of PCD compared to fascia closure and cut-down is to the authors unknown, but many centers (including present study centers) has begun using PCD, which appears to limit the implication of the results of the present RCT. The choice of arterial closure technique used should be a matter for the individual centers and operating surgeons to choose. The authors believe that there is still a role for fascia closure and cut-down technique despite the introduction of PCD into clinical practice since these open techniques remain as common rescue techniques when PCDs fail to achieve adequate hemostasis [21]. The success rate of PCD and fascia closure was in a systematic review of both RCTs and observational studies 63–100% for PCD and 87–99% for fascia closure. [22]
The results of the present multicenter RCT are intriguing but there are a few limitations to consider, such as that the standard dressing used varied by vascular center, and the fact that neither the patients nor treating hospital staff were blinded to allocated wound treatment during in-hospital care. However, the present study also has several strengths. First, the multicenter RCT study design increases generalizability of study results. Secondly, the inclusion and separate statistical analysis of both uni and bilaterally operated incisions, with bilateral incisions receiving both the NPWT and standard dressing, respectively, increases statistical power and minimizes potential confounding [23]. The high proportion of bilateral incisions (89.1%), which is higher than anticipated in the power calculation, adds further scientific strength to the results. Finally, the objectivity of the ASEPSIS score with 100% confirmation from microbiological cultures and diagnosis of SSI by staff at the outpatient clinic blinded to the study, adds objectivity to the otherwise subjective diagnosis of SSI.
Conclusions
The SSI incidence after primary intent of fascia closure for EVAR procedures was low. The present multicenter RCT showed no evidence of difference in SSI incidence in these low-risk inguinal incisions when comparing NPWT with standard dressings after EVAR with the primary intent of fascia closure.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00268- 022-06740-5.
Acknowledgements The authors thank Sophia A ˚ gren Witteschus (RN) at the Vascular Diseases Research Unit at Lund University for her assistance in patient data retrieval. The authors thank Talha Butt (MD, PhD) at the Vascular Center Malmo ¨ for illustration of the fascia closure procedure.
Funding Open access funding provided by Lund University. Research funds were received from the Swedish medical research council (2019–00435), the Hulda Almroth Foundation, Skane University hospital, Region Skane, the Swedish government under the ALF agreement and Smith & Nephew (2013) which also provided 100 PICOTM dressings. The funding sources were not involved in study design, analysis, interpretation, writing or submission of the manuscript.
Ethical approval This study was approved by the regional ethical review board in Lund (Registration number 2013/322 and 2016/886).
Informed consent It was obtained from all individual participants included in the study.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons. org/licenses/by/4.0/.
References
1. Groin wound Infection after Vascular Exposure Study G (2021) Groin wound infection after vascular exposure (GIVE) multicentre cohort study. Int Wound J 18(2):164–75.
2. Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ (2011) Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 32(2):101–114
3. Svensson-Bjork R, Zarrouk M, Asciutto G, Hasselmann J, Acosta S (2019) Meta-analysis of negative pressure wound therapy of closed groin incisions in arterial surgery. Br J Surg 106(4):310–318
4. Gombert A, Dillavou E, D’Agostino R Jr, Griffifin L, Robertson JM, Eells M (2020) A systematic review and meta-analysis of randomized controlled trials for the reduction of surgical site infection in closed incision management versus standard of care dressings over closed vascular groin incisions. Vascular 28(3):274–284
5. Antoniou GA, Onwuka CC, Antoniou SA, Russell D (2019) Meta-analysis and trial sequential analysis of prophylactic negative pressure therapy for groin wounds in vascular surgery. J Vasc Surg 70(5):1700–1710
6. Sexton F, Healy D, Keelan S, Alazzawi M, Naughton P (2020) A systematic review and meta-analysis comparing the effectiveness of negative-pressure wound therapy to standard therapy in the prevention of complications after vascular surgery. Int J Surg 76:94–100
7. Norman G, Shi C, Goh EL, Murphy EM, Reid A, Chiverton L et al (2022) Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 4:CD009261
8. Hasselmann J, Ku ¨hme T, Bjo ¨rk J, Acosta S (2015) Incisional negative pressure wound therapy in the prevention of surgical site infection after vascular surgery with inguinal incisions: rationale and design of a randomized controlled trial (INVIPS-Trial). Surg Sci 6:562–571
9. Trinidad B, Rybin D, Doros G, Eslami M, Tan TW (2019) Factors associated with wound complications after open femoral artery exposure for elective endovascular abdominal aortic aneurysm repair. Int J Angiol 28(2):124–129
10. Montan C, Lehti L, Holst J, Bjorses K, Resch TA (2011) Shortand midterm results of the fascia suture technique for closure of femoral artery access sites after endovascular aneurysm repair. J Endovasc Ther 18(6):789–796
11. Larzon T, Roos H, Gruber G, Henrikson O, Magnuson A, Falkenberg M et al (2015) Editor’s choice. a randomized controlled trial of the fascia suture technique compared with a suturemediated closure device for femoral arterial closure after endovascular aortic repair. Eur J Vasc Endovasc Surg. 49(2):166–73
12. Wilson AP, Treasure T, Sturridge MFG, Gru ¨neberg RN (1986) A scoring method (ASEPSIS) for post-operative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1:311–313
13. Centers for disease control and prevention. Surgical Site Infection (SSI) Events, Chapter 9. [INTERNET]. 2022. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
14. Szilagyi DE, Smith RF, Elliott JP, Vrandecic MP (1972) Infection in arterial reconstruction with synthetic grafts. Ann Surg 176(3):321–333
15. Hasselmann J, Bjork J, Svensson-Bjork R, Butt T, Acosta S (2020) Proposed classification of incision complications: analysis of a prospective study on elective open lower-limb revascularization. Surg Infect (Larchmt) 21(4):384–390
16. Fisher RA (1925) Statistical methods for research workers. Oliver and Boyd, Edinburgh
17. Pettersson J, Daryapeyma A, Gillgren P, Hultgren R (2017) Aortic graft infections after emergency and non-emergency reconstruction: incidence, treatment, and long-term outcome. Surg Infect (Larchmt) 18(3):303–310
18. Vierhout BP, Pol RA, El Moumni M, Zeebregts CJ (2017) Arteriotomy closure devices in EVAR, TEVAR, and TAVR: a systematic review and meta-analysis of randomised clinical trials and cohort studies. Eur J Vasc Endovasc Surg 54(1):104–115
19. Gimzewska M, Jackson AI, Yeoh SE, Clarke M (2017) Totally percutaneous versus surgical cut-down femoral artery access for elective bifurcated abdominal endovascular aneurysm repair. Cochrane Database Syst Rev. 2:CD010185
20. Antoniou GA, Antoniou SA (2021) Editor’s choice - percutaneous access does not confer superior clinical outcomes over cutdown access for endovascular aneurysm repair: meta-analysis and trial sequential analysis of randomised controlled trials. Eur J Vasc Endovasc Surg 61(3):383–394
21. Majid K, Anwar MA, Shepherd A, Malina M, Hussain T (2019) Effectiveness of fascial closure technique following percutaneous endovascular aneurysm repair. Ann R Coll Surg Engl 101(1):14–16
22. Karaolanis G, Kostakis ID, Moris D, Palla VV, Moulakakis KG (2018) Fascia suture technique and suture-mediated closure devices: systematic review. Int J Angiol 27(1):13–22
23. Dell-Kuster S, Droeser RA, Schafer J, Gloy V, Ewald H, Schandelmaier S et al (2018) Systematic review and simulation study of ignoring clustered data in surgical trials. Br J Surg 105(3):182–191
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the World J Surg (2022) 46:3111–3120 by Wound World.


