Introduction
Sternal wound infection following cardiac surgery is a relatively rare but serious complication associated with increased morbidity, mortality and cost [1, 2]. There are few, generally accepted, consensus prevention guidelines for this potentially fatal surgical complication, and clinical practice is often not always supported by high-quality data [3].
The reported incidence of SWI varies widely (2–10%), with a low incidence in studies of deep SWI excluding superficial and donor site infections. Variation in incidence is probably due to the various case definitions, classifications, geographical locations, hospital settings,duration of follow-up and time of publication [4–7]. In Saudi Arabia and the MENA region, the few published reports revealed an incidence between 4 and 10% [8–10].
Published predictors of SWI encompassed all stages of surgical care: preoperative, intraoperative and postoperative. This includes risk factors such as pre-morbidities, elective versus urgent procedures, standard of operation room aseptic and surgical techniques, type of CABG graft conduit, and environment of both operation and recovery rooms. The postoperative course, need for reoperation and blood transfusion, length of intensive care stay and duration of mechanical ventilation were recorded. Additionally, as most SWI cases are diagnosed after discharge from the hospital, the quality of home and outpatient wound care is a reported predictor [11, 12]. The best preoperative predictive index is a 3-point score published by Friedman and colleagues utilizing a large database of 4987 patients who underwent CABG surgery [13].
Recently, Emilo Bouza and associates published a multidisciplinary consensus guideline that included the following proven intervention to reduce SWI: glycaemic control, smoking cessation, weight reduction, incision site preparation with depilatory cream or electric razor to remove the hair and chlorhexidine rather than povidonebased products, screening and eradication of S. aureus, and systematic decontamination when time or other reasons preclude screening. S. aureus should be treated with nasal mupirocin and chlorhexidine for the skin [3].
Modern infection prevention and control practices,including care bundles, have reduced the rates of SWI, but their implementation and enforcement is an ongoing challenge. Despite the abovementioned consensus preventive interventions, we noted variance in our rates of SWI, and the process was out of control in the SWI control chart. Following the implementation of several quality improvement projects, we conducted this focused study to further examine the local risk factors, quantify their relative impact and prioritize them accordingly.
Our objective was to identify possible preventive interventions and to evaluate the effectiveness of the current interventions in reducing the incidence of SWI. The prespecified hypothesis was that a range of specific preoperative and environmental factors would explain the incidence of SWI in our setting. It is generally accepted that SWI rates above 2% are an indication of inadequate surgical practice. This is particularly important in deep sternal wound infections (mediastinitis) given their high mortality rate of 10–40%. The heralding era of value-based purchasing and potential non-reimbursement of SWI, which has already happened elsewhere, will add to the provider’s costs associated with long hospital stays and complex therapy [7].
Methods
Study design
We used a matched case–control design to compare the exposure of patients who underwent major cardiac surgery to a range of risk factors, chosen based on hypothesis, and who went on to develop SWI (cases) with those from the same source population who did not (controls). The source population is an open cohort of patients who underwent cardiac surgery during the defined time period 2017–2020.The matching ratio was 1:3, and cases were matched to control for unmeasured confounders. Explanatory covariates included a range of preoperative, intraoperative and postoperative risk factors, which were chosen from root cause analysis, brain storming and published literature of known predictors of SWI. Institutional approval to conduct the study was obtained.
Setting
The Saud Al-Babtain Cardiac Centre has served the Eastern Province of Saudi Arabia and neighbouring areas since 2001 with a total capacity of 64 beds, and it is located within the 500 bed Dammam Medical Complex.
The Cardiac Surgery database (Dendrite version 1.7) was interrogated in November 2021, and all patients who underwent major cardiac surgery, including CABG, valve replacement, and repairs of all kinds (ICD-10: PCS 012, 02R, 02Q), between 1 January 2017 and 31 December 2020 were recruited as the source population. Both exposure and outcome data were available at the time of data collection. We had no comorbid or gender restrictions. Exclusion criteria: age <18 years, isolated pericardial surgery, short procedure, and cardiac surgery without full median sternotomy.
Participants
Cases of SWI between 2017 and 2020 were ascertained through the Health Care Associated Infection Surveillance records. The cases were diagnosed according to the CDC/NHSN case definition, which includes total SWI: superficial and deep infections. The controls were selected from the same source population. Hospitalbased controls were deemed appropriate and convenient, as they best represent the catchment population of the hospital, and the collection of exposure variables would be more convenient. The case definition is robust, and the collection of variables from hospital records reduced the likelihood of measurement and recall bias.
We initially planned to select 4 controls who did not develop SWI after their cardiac surgery for every case who did. This ratio and the matching were chosen to maximize the power and efficiency of detecting the effect estimates of the exposure covariates’ odds ratio. Additionally, matching allowed control of unmeasured confounders and convenient sampling of the controls. However, we obtained only randomly selected controls to cases at a ratio of 3:1 because of the lack of enough matched female controls in our study population. Independent of exposure, we selected and individually matched controls for sex, age and time of surgery. Gender was randomly matched first, then age±2 years, and finally time of surgery±2 years [14, 15].
Variable sources and measurement
The outcome variable was binary, with the case of SWI or control coded as indicator variables one and zero, respectively. Our candidate explanatory variables included 23 covariates, both continuous and categorical. Some are derived variables from the raw data. In addition, we scaled some continuous variables to clinically relevant groups. Additionally, we added two design variables, strata and time, to allow conditional logistic regression analysis. The continuous variables were age, inpatient days, HbA1c, weight, BMI, EuroSCORE II, perioperative blood glucose, haemoglobin, white cell count and surgery time. The categorical variables included sex, mortality, procedure, graft conduit, diabetes, smoking, pre-morbidity, antibiotic prophylaxis, infection control supply status, enrolment in an ongoing antibiotic prophylaxis study, environmental issues in the operating room, surgeon ID code, blood transfusion, and re-exploration.
The sources and measurements of our variables included the dendrite database, where data were retrieved directly from medical and laboratory records, and infection control surveillance records were obtained separately. Tere are dedicated personnel who enter clinical and laboratory data into the database. Laboratory data were obtained from a single inhouse laboratory, observing the CLSI standards, which did not change its methods within the study period. Additionally, the infection control staff is well trained in surveillance methods and uses a robust inpatient and outpatient surveillance system based on the NHSN case definition. There were no differences between cases and controls in the way of measuring the outcome and exposure variables. Potential confounders included HA1c, weight, BMI, pre-morbidities, blood transfusion and re-exploration. The anticipated effect modifiers were age and sex.
Mitigation of potential sources of bias
Cases of SWI were ascertained within the continuous surveillance system for hospital-acquired infections using internationally recognized methods and documentation guidance (NHSN/CDC), the case is defined as SWI related to surgery within 30–90 days from the operation, and include superficial, subcutaneous, and organ space infections. The latter uses both clinical and microbiological criteria to confirm the diagnosis and is concurrent during hospital stay with outpatient follow up. Tis makes misclassification unlikely. Additionally, the exposure variables consist of clinical and laboratory data documented in the participants’ medical records and hence are not subject to recall bias. Furthermore, we chose cases and controls completely independent of exposure. In addition, the design of our study and source of variables did not have issues of potential participation and non-participation, as we had no questionnaires and, of course, we had no follow up-related potential sources of bias in this case–control design. Therefore, overall, the potential for selection and misclassification bias is small. Thus, we assume that neither the external nor infernal validity of our study is likely to be compromised.
Sample size
The sample size was determined by the number of cases during the study period. There were 51 cases and 153 matched controls, which made our total study size 204. Additionally, we entered this sample number into the CDC EpiInfo sample size calculator. It calculated a detection ability of an OR difference of 2.60 for population exposure of 20% and 6.06 for exposure of 2% at 80% power and 5% alpha.
Statistical methods
Statistical Analysis Software (SAS)–version 9.4 was used to analyze all the data after importation from the final Excel workbook, where they were stored after collection from the sources. We used the t-test, ChiSq-test and conditional logistic regression to compare patients with SWI and their matched controls for selected perioperative risk factors.
We visualized, cleaned, corrected, recoded, scaled, and filled-in missing values by multiple imputation, as data were assumed to be missing at random (MAR). After initially filling in missing values by reviewing the sources, the remaining missing values were HbA1c (39/204, 19%) and surgery time (25/204, 12%). No missing outcome variables. Multiple imputation was used due to the relatively large missing values. Sensitivity testing with incomplete data, single imputation, and full information maximum likelihood (FIML) was used for comparison.
Then, we obtained descriptive statistics and a correlation matrix and compared the distribution of covariates between cases and controls. Finally, we fitted several univariate and a final multivariate logistic regression model. We used conditional analysis in all models.
Descriptive statistics included summaries and plots of all the exposure variables using univariate and frequency procedures for continuous and categorical variables, respectively. Additionally, differences in exposure between cases and controls were compared with t-tests and Chi-sq tests. Furthermore, a correlation matrix was obtained to detect any potential colinear variables, cut of (r= >0.7).
Univariate analysis yielded variables associated with SWI, and those with p values<0.10 were selected to progress to the final multivariate logistic model. We entered all determinant variables individually into 3 blocks. Confounding and interactions were identified. The total number of covariates was 23, and the final complete multivariate model included 12 significant covariates with p<0.5 from univariate analysis, including confounders. Two colinear variables were removed. Model diagnostics were used to assess and compare the models and their goodness of ft (GOF).
Results
Among 985 eligible patients who underwent major cardiac surgery with median sternotomy, two were excluded because of age under 18 years. There were 51 cases of SWI, no exclusive donor site infection, and 153 randomly selected matched controls from the remaining 932 patients who met the inclusion criteria. Thus, the total sample size was 204 patients. The distribution of missing data was stable among groups, and the maximum was 16% and 19% in the HbA1c variable among cases and controls, respectively, while the surgery time missing values were identical in each group at 12%. The clinical and demographic characteristics of the participants are summarized in Table 1.
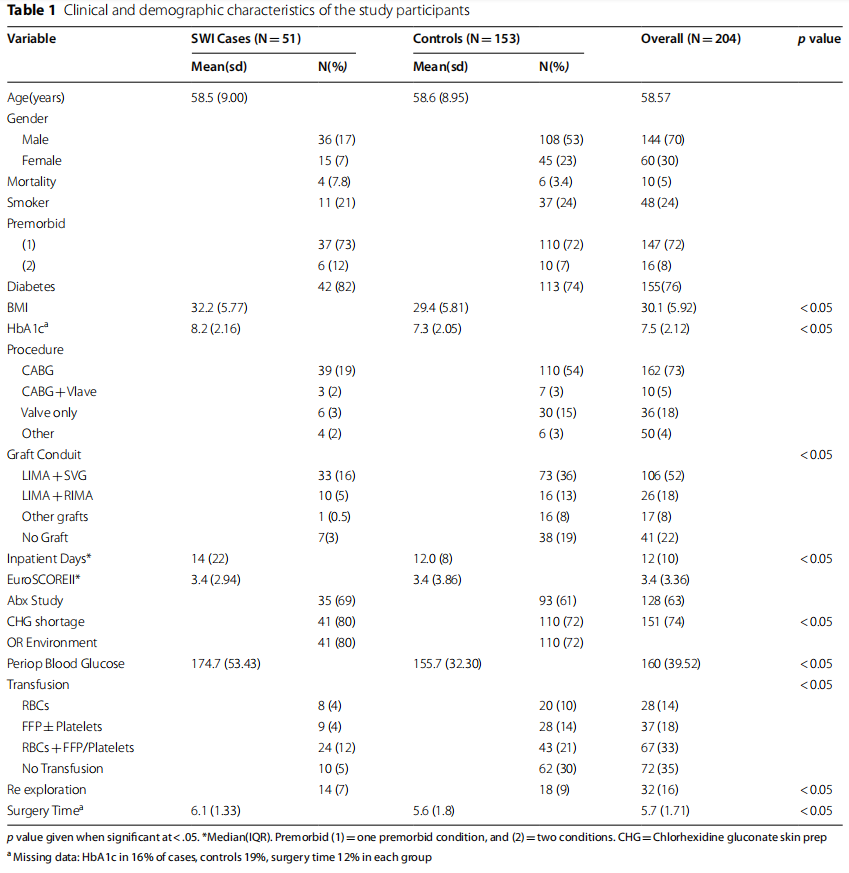
We initially considered the following variables: preoperative and total inpatient days, mortality status, surgical procedure, graft conduit, diabetes status, HbA1c, BMI, blood glucose within 24 h of surgery, weight, smoker status, premorbid status, EuroSCORE-II, participation in an ongoing antibiotic prophylaxis study, shortage of chlorhexidine gluconate skin prep (CHG), operating room (OR) environmental issues, surgeon ID, Hb, WBC, blood transfusion, re-exploration, and duration of surgery. The variables associated with SWI in univariate analysis, with p values<0.10 and unadjusted estimates, are shown in Table 2.
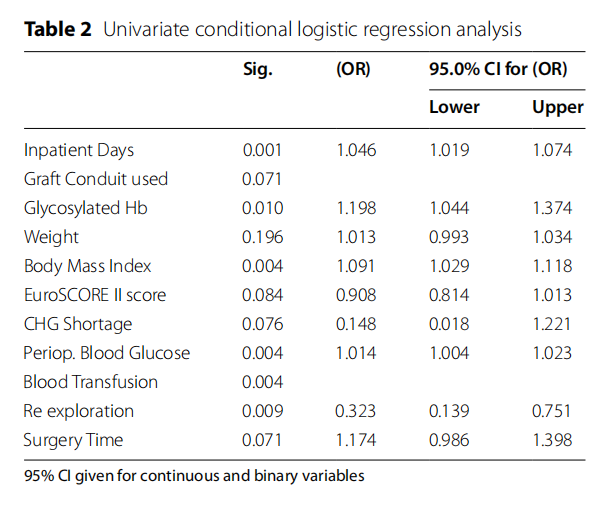
Eight variables were removed at this stage: preoperative hospital days, mortality status, smoking status, surgeon ID, Hb and WBC, all with a p value>0.10. The procedure and operating room environment were excluded because of collinearity with the graft conduit and the shortage of CHG, respectively.
In Table 3, we display the final adjusted estimates associated with SWI. Eleven variables were included in the global final multivariate conditional logistic model: inpatient days, graft conduit, HbA1c, weight, BMI, EuroSCORE II, shortage of CGH, perioperative blood glucose, blood transfusion, re-exploration and surgery time.
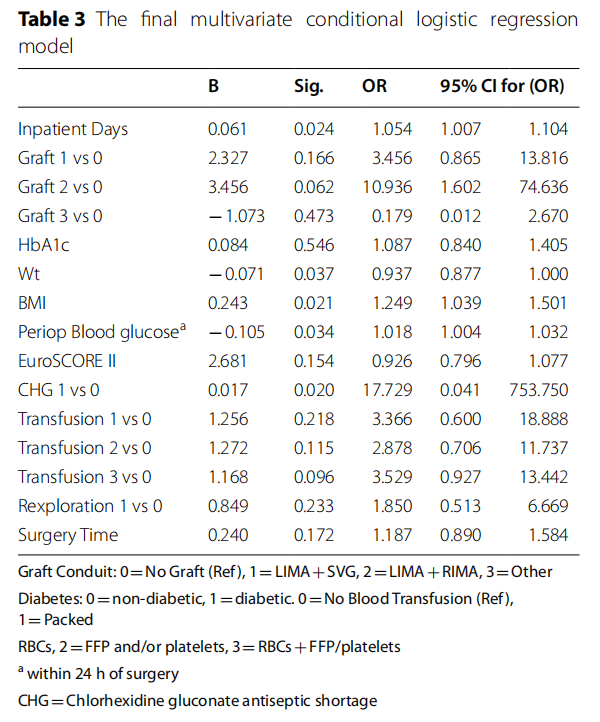
Scaled continuous variables along clinically relevant groups: HbA1c percentage<6>6<10 and>10%, BMI>30 kg/M2 and<30 kg/M2, EuroSCORE<2,>2<6 and>6 were entered in the model. They did not reveal more information than their respective scale forms except BMI>30 kg/M2.
We included the following confounders in the final model: weight is a confounder of BMI, blood transfusion confounded re-exploration, all at>10% change of the regression coefficient size compared to the univariate values. Additionally, the matching variables age and gender significantly modified the effects of graft and procedure variables.
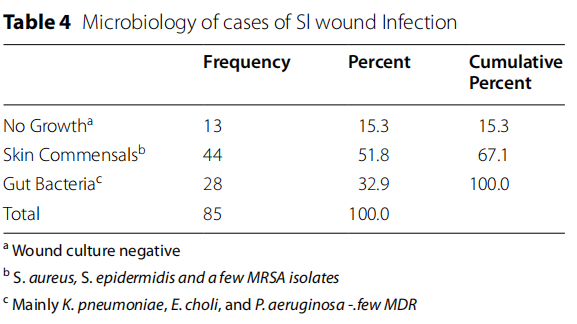
Eighty-five microbiology samples obtained from SWI cases revealed skin commensal (44/52%), gut bacteria and Pseudomonas aeruginosa (28/33%), and no growth in (13/15%). Commensals were mainly Staphylococcus species, including a few cases of MRSA, while gut bacteria were mainly Klebsiella pneumoniae, Escherichia Choli and Pseudomonas aeruginosa, as detailed in Table 4.
The Hosmer and Lemeshaw test was insignificant at a p value of<0.26. Additionally, Cox & Snell and Nagelkerke GOF R square were 0.28 and 0.41, respectively. All suggest that our final logistic model is a good ft for the data. In addition, the overall classifying accuracy of the model is good, with an ROC of 84%.
As the missing values were relatively high for HbA1c (60/204, 19) and Operation time (25/204, 12%) variables and distributed between cases and controls, 16% and 20% for HbA1c and 12% each for operation time, respectively. Nonetheless, we obtained 2 models using single, mean/ median imputed data sets and FIML analyses. All produced comparable models to our final model shown in Table 3. Alternative GOF tests were used for comparison due to well-recognized short comings of the Hosmer Lemeshaw GOF test as described by Allison [16]. Skutel, deviance and Pearson, all did not reject our model at Skutel 0.28, McFadden R-square 0.25, Deviance 0.27 and Pearson 0.45. The model did not need adding nonlinearity or interaction terms to [16].
Discussion
Our study revealed that inpatient days, graft conduit, HbA1c, weight, BMI, EuroSCORE II score, perioperative blood glucose, blood transfusion, re-exploration and surgery time are associated with developing SWI.
Our results are consistent with previously published data. Despite implementation of the surgical wound infection prevention bundle, modifiable risk factors were still identified as predictors in our model. For example, perioperative blood glucose predicted SWI despite being within the recommended range of 140–180 mg/dl (mean 174.7 mg/dl in SWI cases and 155.7 mg/dl in controls), which suggests that tighter control, rather than moderate, is required in selected patient groups than recommended for the perioperative level by authoritative guidelines. In addition, innovative multidisciplinary implementation of proven intervention is required. [5, 6, 10, 17, 18]
Risk factors not reaching statistical significance, which were previously consistently reported in the literature as predictors, were most likely not identified because of the underpower of our study and/or bias towards the null due to matching with 3 variables. These include duration of preoperative admission, smoker and diabetes status, and premorbid conditions. In addition, we chose 3 variables: shortage of CHG skin prep products, participation of an ongoing surgical antibiotic prophylaxis, and presence of operation room pressure, temperature and/ or humidity concerns around the time of surgery (within 72 h), all of which did not predict SWI in the final model. An exploratory analysis using an unsplit imputation data set (not shown) revealed that all these factors were significant except smoking and operation room environmental issues with a larger sample size.
Pathogens isolated in SWI were in line with previously published data from Saudi Arabia and elsewhere, and the prevalence of multidrug resistant organisms was small [9].
The strength of this study is evaluating 23 potential risk factors of SWI in a good ft logistic regression model. Twelve were associated with SWI in univariate regression analysis, and 8 remained significant or trending in the final model. This is similar to the risk factors published in Western European countries. In addition, shortages in CHG skin prep supplies, and hence use of Bovidone-based alternatives during the COVID-19 pandemic, were associated with a 16-fold increase in SWI (OR 17.73), McNamar’s test p < 0.000, but the 95% CI was too wide. Our study is an addition to the few published studies on determinants of SWI in the MENA region and is likely to be generalizable.
Weaknesses of this work include the relatively small sample size (determined by the study period needed). The low sensitivity of the OR environment and CHG shortage variables, as they are not strictly individuallevel variables, did not show significant effects. Additionally, we did not study important predictors of SWI due to nonavailability, which is generally less studied. The latter include variation in operative techniques, hemostasis materials, diathermy use and postdischarge home wound care. These are likely to be sources of residual confounding.
Practically, this study helped to inform an ongoing quality improvement project in our setting by prioritizing the tacking of certain risk factors. Additionally, we highlighted the need for improved perioperative glycemic control, review selection for bilateral internal memory grafts and rationalize blood transfusion, all recognized causes of delayed sternal healing and risk of SWI. Some centers have established dedicated teams and multidisciplinary protocols to supervise perioperative glycemic control. This should, however, be individualized and not impact critically ill patients who would sufer more from hypoglycemia, as shown in the NICESUGAR study [19]. However, the real challenge here is likely to be the implementation of multidisciplinary protocols regardless of the blood glucose target, which remain controversial [19–21].
In conclusion, the advent of value-based contracting and nonremuneration of surgical wound infections should focus healthcare providers’ minds on the reduction of SWI and hence the need for more robust multidisciplinary research and evidence-based prevention efforts, specially on the poorly studied potential risks mentioned above. Our study suggested that the implementation of preventive interventions for SWI need to be improved.
Acknowledgements
I am grateful to Josephine Grace Chua, Rania Alshaf, and Ayat Buali for for retrieving data from the Dendrite database, the laboratory and Infection Control records respectively. I am also grateful to the staf from the Cardiac Surgery and Quality and Patient Safely departments for participating in several brain storming and quality improvement projects that informed this study. I appreciate the helpful comments of 5 anonymous reviewers who helped improve a previous draft of this paper.
Author contributions
AI conceived the study, obtained the data, performed the analysis and wrote the manuscript. The author read and approved the fnal manuscript.
Funding
No funding.
Availability of data and materials
At request from the corresponding author.
Declarations
Ethics approval and consent to participate
Internal Review Board of the SBCC approval was obtained.
Consent for publication
Not applicable, as data were retrieved from an on-going primary cohort in which consent was already obtained in the past.
Competing interests
The authors declare no competing interests.
Received: 16 August 2022 Accepted: 5 January 2023
Published online:16 January 2023
References
1. El Oakley RM, Wright JE. Postoperative mediastinitis: Classification and management. Ann Thorac Surg. 1996;61:1030–6.
2. Sears ED, Wu L, Waljee JF, Momoh AO, Zhong L, Chung KC. The impact of deep sternal wound infection on mortality and resource utilization: a population-based study. World J Surg. 2016;40(11):2673–80. https://doi. org/10.1007/s00268-016-3598-7. (PMID: 27283188).
3. Bouza E, de Alarcón A, Fariñas MC, Gálvez J, Goenaga MÁ, Gutiérrez-Díez F, Hortal J, Lasso J, Mestres CA, Miró JM, Navas E, Nieto M, et al. Prevention, Diagnosis and Management of Post-Surgical Mediastinitis in Adults Consensus Guidelines of the Spanish Society of Cardiovascular Infections (SEICAV), the Spanish Society of Thoracic and Cardiovascular Surgery (SECTCV) and the Biomedical Research Centre Network for Respiratory Diseases (CIBERES). J Clin Med. 2021;10(23):5566. https://doi.org/10.3390/ jcm10235566. (PMID:34884268;PMCID:PMC8658224).
4. Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, Cohen O, Milo S. Surgical site infection rates following cardiac surgery: the impact of a 6-year infection control program. Am J Infect Control. 2005;33(8):450–4. https://doi.org/10.1016/j.ajic.2005.07.002. (PMID: 16216658).
5. Lepelletier D, Perron S, Bizouarn P, Caillon J, Drugeon H, Michaud JL, Duveau D. Surgical-site infection after cardiac surgery: incidence, microbiology, and risk factors. Infect Control Hosp Epidemiol. 2005;26(5):466–72. https://doi.org/10.1086/502569. (PMID: 15954485).
6. Fowler VG Jr, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112(9 Suppl):I358–65. https://doi.org/10.1161/CIRCULATIO NAHA.104.525790. (PMID: 16159846).
7. Lazar HL, Salm TV, Engelman R, Orgill D, Gordon S. Prevention and management of sternal wound infections. J Thorac Cardiovasc Surg. 2016;152(4):962–72. https://doi.org/10.1016/j.jtcvs. 2016.01.060. (Epub 2016 Aug 8 PMID: 27555340).
8. Elassal AA, Al-Ebrahim KE, Al-Radi OO, Jabbad HH, Eldib OS. Sternal wound complications: objective reclassification and surgical reconsideration. Heart Surg Forum. 2020;23(1):E076–80. https://doi.org/10.1532/hsf. 2649. (PMID: 32118548).
9. Majid FM, Buba FM, Barry M, Alsharani F, Alfawzan F. Incidence, types and outcomes of sternal wound infections after cardiac surgery in Saudi Arabia. A retrospective medical chart review. Saudi Med J. 2020;41(2):177–82. https://doi.org/10.15537/smj.2020.2.24843. (PMID: 32020152; PMCID: PMC7841641).
10. Hosseinrezaei H, Rafei H, Amiri M. Incidence and risk factors of sternal wound infection at site of incision after open-heart surgery. J Wound Care. 2012;21(8):408–11. https://doi.org/10.12968/jowc. 2012.21.8.408. (PMID: 22885314).
11. Sexton DJ. Postoerative mediastinitis after cardiac surgery. In: Allyson B, editor. Up to date. 2022. Retrieved from https://www.uptodate.com/ contents/postoperative-mediastinitis-after-cardiac-surgery?source=histo ry_widget.
12. Jonkers D, Elenbaas T, Terporten P, Nieman F, Stobberingh E. Prevalence of 90-days postoperative wound infections after cardiac surgery. Eur J Cardiothorac Surg. 2003;23(1):97–102. https://doi.org/10.1016/s1010- 7940(02)00662-0. (PMID: 12493512).
13. Friedman ND, Bull AL, Russo PL, Leder K, Reid C, Billah B, Marasco S, McBryde E, Richards MJ. An alternative scoring system to predict risk for surgical site infection complicating coronary artery bypass graft surgery. Infect Control Hosp Epidemiol. 2007;28(10):1162–8. https://doi.org/10. 1086/519534. (Epub 2007 Aug 3 PMID: 17828693).
14. Costanza MC. Matching. Prev Med. 1995;24(5):425–33. https://doi.org/10. 1006/pmed.1995.1069. (PMID: 8524715).
15. Mansournia MA, Hernán MA, Greenland S. Matched designs and causal diagrams. Int J Epidemiol. 2013;42(3):860–9. https://doi.org/10.1093/ije/ dyt083.PMID:23918854;PMCID:PMC3733703.
16. Allison PD. Measures of Fit for Logistic Regression. 2014. https://support. sas.com/resources/papers/proceedings14/1485-2014.pdf.
17. Cotogni P, Barbero C, Rinaldi M. Deep sternal wound infection after cardiac surgery: evidences and controversies. World J Crit Care Med. 2015;4(4):265–73. https://doi.org/10.5492/wjccm.v4.i4.265.PMID:26557 476;PMCID:PMC4631871.
18. Dogra P, Jialal I. Diabetic Perioperative Management. [Updated 2022 Jun 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54 0965/
19. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97.
20. Hweidi IM, Zytoo AM, Hayajneh AA. Tight glycaemic control and surgical site infections post cardiac surgery: a systematic review. J Wound Care. 2021;30(Sup12):S22–8. https://doi.org/10.12968/jowc. 2021.30.Sup12.S22. (PMID: 34882005).
21. Reddy P, Duggar B, Butterworth J. Blood glucose management in the patient undergoing cardiac surgery: a review. World J Cardiol. 2014;6(11):1209–17. https://doi.org/10.4330/wjc.v6.i11.1209.PMID:25429 332;PMCID:PMC4244617.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the Isaac Journal of Cardiothoracic Surgery (2023) 18:28 by Wound World.


