Biofilm formation establishes by bacterial colonisation in wound beds through the attachment of pioneer microbes, followed by microcolonies that produce extracellular polymer substances (EPS) (Flemming and Wingender, 2010). Following colonisation, bacteria adhere to host tissue and if not cleared promptly by the host immune response, biofilm formation frequently ensues and is the primary, causative factor in hard-to-heal chronic wounds. The efficacy of antibiotic therapy regimens may be decreased by the presence of biofilms and multidrug-resistant (MDR) bacteria.
The National Institutes of Health (NIH) estimate that biofilms play a role in over 80% of bacterial infections (NIH, 2022). It is estimated there are over 14 million biofilm infections per year in the US with over 350,000 biofilm-related deaths (Wolcott et al, 2010). A study published by the UK’s National Biofilms Innovation Centre (NBIC) determined the economic impact of biofilms globally to be over $5 trillion USD per year (Cámara et al, 2022). The analysis was divided into sectors of health, homecare, food, water and energy. The medical and human health sector accounted for $386.8 billion with an estimated $281 billion associated with biofilms and wounds annually (Cámara et al, 2022).
Given the preponderance of biofilms in chronic wounds (78.2%) (Malone et al, 2017), the impact on public health, and associated expenditures, it is important to explore antibiofilm strategies that can prevent the formation and persistence of biofilm in wounds. These strategies would consequently decrease the incidence of chronic wounds and improve wound healing. Use of preclinical biofilm models to assess the antibiofilm efficacy of wound dressings is a prerequisite in identifying new technologies, which can improve outcomes in hard-to-heal wounds.
In vitro biofilm test methods
The focus on bacterial biofilms has rapidly increased in the past 20 years due to the recognition of the role they play in most human infections. They have typically been studied using in vitro models in the laboratory such as microtiter plates, which are a closed, static model, and dripflow cells or CDC reactors, which are open, dynamic systems. These simplified models have contributed greatly to fundamental knowledge of biofilm formation, physiology, and structure. Benefits of these models include low cost, easy set-up, ability to standardise, and scalability for high throughput screening (Guzmán-Soto et al, 2021). However, there exists a translational disconnect in this type of research due to a lack of appreciation for the multiple factors, both microbial and host-derived, that influence disease outcomes in the clinic.
More recently, in vivo (animal) models have been developed that recapitulate many aspects of host-pathogen interactions, especially for medical device-related infections (Guzmán-Soto et al, 2021). Commonly used animal models include rodents (mice or rats) or larger animals, such as the pig (Ganesh et al, 2015). The Wound Healing Society has recommended a porcine skin model for preclinical studies of wounds due to its similarity to human pathophysiology (Gordillo et al, 2013). In vivo models often slow the development and regulatory process of new, effective therapies for the treatment of chronic wounds due to the lack of high throughput, cost, and difficulties in applications of wound dressings and sampling. Improvements to approaches for in vitro and in vivo biofilm models have been suggested recently including considerations for translation of the research to the clinic and the native microenvironment of the biofilm (Guzmán-Soto et al, 2021; Highmore et al, 2022). There is a high demand to develop alternative, reproducible, and high throughput models that can be used to assess the antimicrobial and antibiofilm effectiveness of topical wound dressings.
Ex vivo wounded skin models
Ex vivo human (Ashrafi et al 2018; Yoon et al, 2019; Highmore et al, 2022; Siler et al, 2022) and porcine skin (Phillips et al 2013; Yang et al 2013; Ueck et al 2017; Alves et al, 2018; Andersson et al, 2021) models have been developed and used to assess the efficacy of antimicrobial wound dressings. These models, derived from living tissue, bridge the gap between in vitro and in vivo studies providing a biologically relevant biofilm environment to assess wound dressings. Ex vivo models are more predictive of clinical success compared with in vitro experiments, while remaining more cost-effective and an alternative to live animal testing (Phillips et al, 2013). Ex vivo models enable evaluations of the antibiofilm efficacy and cytotoxicity of therapeutic formulations, dressings, and devices. They may also be used to investigate immune response and wound healing (Eberlin et al, 2020; O’Connor et al, 2020). A review of wound healing research from 1993 to 2017 determined less than 3% of wound research models were ex vivo models (Parnell et al, 2019).
Ex vivo porcine skin biofilm models
Many of the developed ex vivo wound models are porcine skin-derived due to similarities with human skin and the recommendation of in vivo porcine skin models for the development of wound dressings. Porcine skin has been demonstrated to be physiologically similar to human skin, and is readily available and inexpensive (Seaton et al, 2015). Yang et al (2013) developed a novel ex vivo porcine partial-thickness wound skin explant model to study mature bacterial biofilms. This model was an improvement over in vitro biofilm models as it incorporated host proteins and extracellular matrix components. Commonly biofilm-forming bacteria in wounds, Pseudomonas aeruginosa and Staphylococcus aureus, developed mature biofilms that had increased tolerance to antimicrobials. In a follow-up study, Phillips et al (2013) used this porcine skin biofilm model to determine the efficacy of several commercially available wound therapies or dressings against mature Pseudomonas aeruginosa biofilm. They further correlated the efficacy findings using an in vivo pig burn wound model, demonstrating a correlation between the ex vivo and in vivo models.One weakness of this ex vivo porcine model is the sterilisation process using chlorine gas was toxic to the skin. While it is possible to evaluate the formation of biofilms in a biologically relevant context, the ability to measure the host’s response to the bacteria or potential biocompatibility to the wound dressing was lacking.
Alves et al (2018) further developed an ex vivo porcine skin wound biofilm model using a custom burn wound array device. The model was developed to support high-throughput studies of biofilm formation, bacterial virulence gene expression, and potential phage therapy to control biofilm formation by Staphylococcus aureus. Their findings noted differences in Staphylococcus aureus virulence factor production and biofilm formation not obtained in abiotic (in vitro) models. This further highlights the importance of using ex vivo skin substrate for growth and biofilm formation in translating wound dressing efficacy findings to the clinical setting. Furthermore, this model has been demonstrated to be reproducible and was further developed commercially by Perfectus Biomed Group achieving ISO 17025 certification and UKAS accreditation. Likewise, Andersson, et al. developed an ex vivo porcine wound infection model using porcine skin, previously frozen, and a burn wounding method to evaluate topical antimicrobial formulations against Staphylococcus aureus and Pseudomonas aeruginosa (Andersson et al, 2021). This study demonstrated both bacterial strains infected the wound surface and deeper regions of the tissue. These burn wound models give flexibility to easily change wound size and depth and are optimal for the application of various topical treatments.
Ex vivo wounded human skin models for biofilm assessment
The development of ex vivo human skin models for evaluation of antibiofilm effectiveness and wound healing represents an experimental model that most closely reproduces the clinical utility of products. The development of ex vivo human skin models to study wound dressings creates a non-live animal alternative for studying human biological response and a testing platform cultured at the air-liquid interface for characterisation of biocompatibility, antimicrobial effectiveness, and wound healing properties. Skin explants from different regions of the body have been characterised previously with abdomen skin following elective abdominoplasty being the predominant (Eberlin et al, 2020). Tissue viability, re-epithelialisation, and epidermal markers such as cytokeratins that are important for healing have been described previously (Pastar et al, 2014; Mendoza-Garcia et al, 2015).
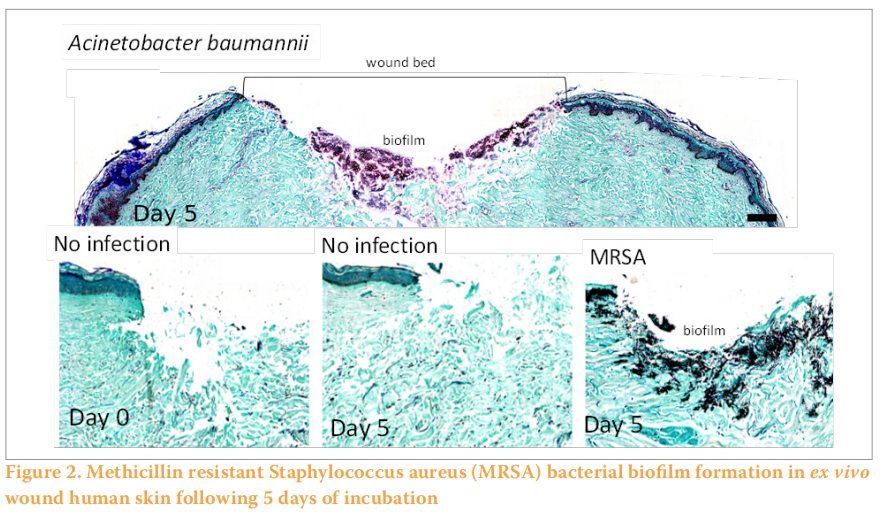
Similar to the ex vivo wounded porcine skin models described previously, an ex vivo wounded human skin model was developed for the characterisation of biofilm formation in wounds (Figure 1) (Siler et al, 2022). Explants are prepared within hours of abdominoplasty surgery and partial-thickness wounds are created and inoculated with bacteria and incubated for several days until mature biofilm formation on the surface and below the wound bed (Figure 2). Both methicillin-resistant Staphylococcus aureus (MRSA) and MDR Acinetobacter baumannii formed biofilms within the wound bed as noted by Gram Twort Staining. This ex vivo wounded human skin biofilm model can be used to study the effectiveness of wound dressings for biofilm reduction, prevention and elimination.
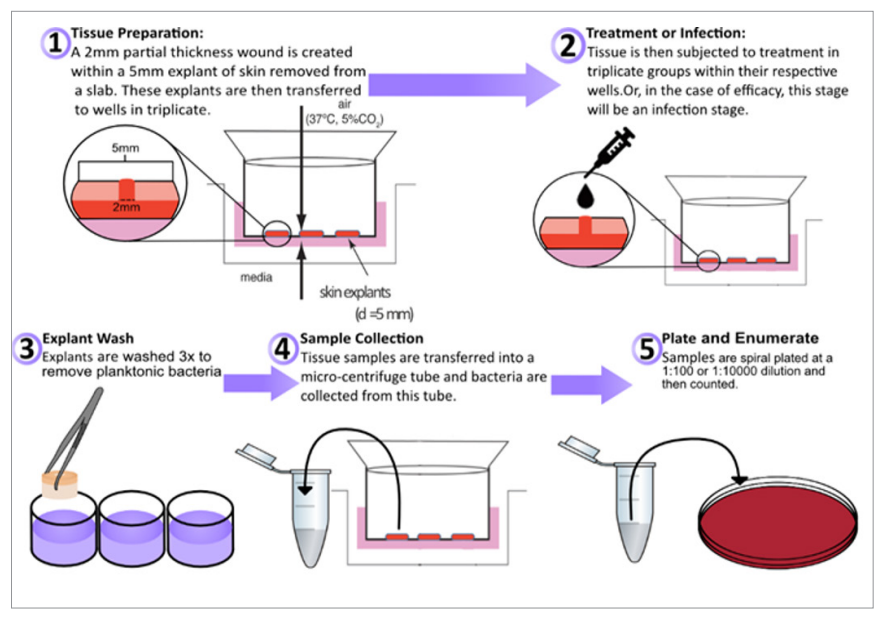
Figure 1. Ex vivo wounded human skin model for wound dressing studies
Ex vivo wounded skin models for wound healing
Another important factor for the assessment of wound dressings is healing. Ex vivo human and porcine skin models have been developed previously to study wound healing through regeneration of the epidermis and stimulation of cell factors such as cytokines and keratinocytes (Pastar et al, 2014; Eberlin et al, 2020). Uek et al advanced these studies further to incorporate preconditioning of ex vivo porcine skin with hyperglycaemia conditions to simulate decreased wound healing under diabetic conditions (Ueck et al, 2017). These studies demonstrated the importance of culture conditions in wound healing test systems.
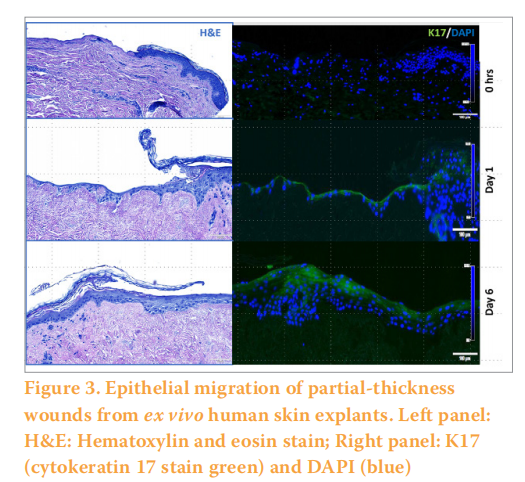
As we and others have described previously, ex vivo wounded human skin explants can be used to determine biocompatibility and promotion of re-epithelialisation (healing) of wound dressings (O’Connor et al, 2020). In addition, cell migration of the epithelium of ex vivo human skin explants can be observed with partial-thickness wounds when cultured with an air-liquid interface can be observed within six days. (Figure 3). These models in the future can accelerate the development of topicals including wound dressings in the clinic.
CONCLUSIONS
Biofilms are estimated to be present in at least 80% of chronic wounds. The microbial biofilms significantly delay antimicrobial effectiveness and wound healing, and the associated expenditures are as high as $281 billion annually. Therefore, it is imperative to explore antibiofilm strategies that can prevent the formation and persistence of biofilm in wounds. The research approach and regulatory process for developing and accessing wound healing dressings for antibiofilm effectiveness are also important. Improvements to preclinical approaches (in vitro and in vivo) to biofilm models are needed. Living tissue (ex vivo) derived from pig and human skin donors is an approach that includes considerations for translation of the research to the clinic and the native microenvironment of the biofilm. These ex vivo wounded skin models allow for the simultaneous assessment of antibiofilm effectiveness and promotion of wound healing. Ex vivo models are more predictive of in vivo and clinical success compared to in vitro (abiotic) experiments while remaining more cost-effective and an alternative to live animal testing. While these models represent high potential for wound dressing research and development, ex vivo models are still contributing to less than 3% of preclinical research. There is a need for continued research for standardisation of ex vivo wounded skin biofilm models to allow for these methods to be recognised in the regulatory and commercial development process for wound healing dressings.
Although in vitro models should reflect the clinical situation as best possible, the more complex a model is, the more variables are introduced, and this compromises the robustness of a test method. Basic in vitro studies are likely to produce the most reproducible data sets and thus relatively small-scale studies can generate good, robust data. At the other end of the scale, the significant variabilities associated with clinical studies (no two patients or wounds are alike) means that a large participant number is typically required to produce reproducible data about a products performance. Consequently, a balance needs to be struck between simplicity and simulating a wound environment as closely as possible.
REFERENCES
1. Alves DR, Booth SP, Scavone P et al (2018) Development of a high-throughput ex-vivo burn wound model using porcine skin, and its application to evaluate new approaches to control wound infection. Front Cell Infect Microbiol 8:196. https://doi.org/10.3389/fcimb.2018.00196
2. Andersson MÅ, Madsen LB, Schmidtchen A, Puthia M (2021) Development of an experimental ex vivo wound model to evaluate antimicrobial efficacy of topical formulations. Int J Mol Sci 22(9):5045. https://doi. org/10.3390/ijms22095045
3. Ashrafi M, Novak-Frazer L, Bates M, et al (2018) Validation of biofilm formation on human skin wound models and demonstration of clinically translatable bacteria-specific volatile signatures. Sci Rep 8(1):9431. https://doi.org/10.1038/s41598-018-27504-z
4. Cámara M, Green W, MacPhee CE, et al (2022) Economic significance of biofilms: a multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 8:42. https://doi.org/10.1038/s41522-022-00306-y
5. Eberlin S, Silva MS da, Facchini G et al (2020) The ex vivo skin model as an alternative tool for the efficacy and safety evaluation of topical products. Altern Lab Anim 48(1):10–22. https://doi. org/10.1177/0261192920914193
6. Flemming HC, Wingender J (2010)The biofilm matrix. Nat Rev Microbiol 8(9):623–33. https://doi.org/10.1038/nrmicro2415
7. Ganesh K, Sinha M, Mathew-Steiner SS et al (2015) Chronic wound biofilm model. Adv Wound Care (New Rochelle) 4(7):382–8. https://doi. org/10.1089/wound.2014.0587
8. Gordillo GM, Bernatchez SF, Diegelmann R, et al (2013) Preclinical models of wound healing: is man the model? Proceedings of the Wound Healing Society Symposium. Adv Wound Care (New Rochelle) 2(1):1–4. https:// doi.org/10.1089/wound.2012.0367
9. Guzmán-Soto I, McTiernan C, Gonzalez-Gomez M, et al (2021)Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 24(5):102443. https://doi.org/10.1016/j. isci.2021.102443
10. Highmore CJ, Melaugh G, Morris RJ, et al (2022) Translational challenges and opportunities in biofilm science: a BRIEF for the future. npj Biofilms Microbiomes 8:68. https://doi.org/10.1038/s41522-022-00327-7
11. Malone M, Bjarnsholt T, McBain AJ et al (2017) The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care 26(1):20–5. https://doi.org/10.12968/ jowc.2017.26.1.20
12. Mendoza-Garcia J, Sebastian A, Alonso-Rasgado T, Bayat A (2015) Optimization of an ex vivo wound healing model in the adult human skin: Functional evaluation using photodynamic therapy. Wound Repair Regen 23(5):685–702. https://doi.org/10.1111/wrr.12325
13. NIH Guide: Research on microbial biofilms. https://tinyurl.com/4zzt59wf (accessed 20 Spetember 2022)
14. O’Connor NA, Syed A, Wong M et al (2020) Polydopamine antioxidant hydrogels for wound healing applications. Gels6(4):39. https://doi. org/10.3390/gels6040039
15. Parnell LKS, Volk SW (2019)The Evolution of animal models in wound healing research: 1993–2017. Adv Wound Care (New Rochelle)
16. Pastar I, Stojadinovic O, Yin NC et al (2014) Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle) 3(7):445–64. https://doi.org/10.1089/wound.2013.0473 8(12):692–702. https://doi.org/10.1089/wound.2019.1098
17. Phillips PL, Yang Q, Davis S et al (2015)Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 12(4):469–83. https://doi.org/10.1111/iwj.12142
18. Seaton M, Hocking A, Gibran NS (2015) Porcine models of cutaneous wound healing. ILAR J 56(1):127–38. https://doi.org/10.1093/ilar/ilv016
19. Siler Z, Peterson M (2022) A wounded ex vivo human skin model to study microbial biofilm development and anti-biofilm therapeutics. In: 31st European Congress of Clinical Microbiology & Infectious Diseases. https://tinyurl.com/yktpwt5n (accessed 20 October 2022)
20. Ueck C, Volksdorf T, Houdek P et al (2017) Comparison of in-vitro and exvivo wound healing assays for the investigation of diabetic wound healing and demonstration of a beneficial effect of a triterpene extract. PLoS ONE 12(1):e0169028. https://doi.org/10.1371/journal.pone.0169028
21. Wolcott RD, Rhoads DD, Bennett ME et al (2010) Chronic wounds and the medical biofilm paradigm. J Wound Care 19(2):45–53. https://doi. org/10.12968/jowc.2010.19.2.46966
22. Yang Q, Phillips PL, Sampson EM et al (2013) Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen 21(5):704–14. https://doi.org/10.1111/ wrr.12074
23. Yoon DJ, Fregoso DR, Nguyen D et al (2019) A tractable, simplified ex vivo human skin model of wound infection. Wound Repair Regen 27(4):421–5. https://doi.org/10.1111/wrr.12712
Acknowledgment We would like to thank Zach Siler, MS for his contributions to this manuscript.
This article is excerpted from the Wounds UK | Vol 18 | No 4 | 2022 by Wound World.


