Introduction
Obesity affected 107.7 (98.7-118.4) million children and 603.7 (588.2-619.8) million adults worldwide in 2015. Globally, China had the highest number of obese children in 2015 (Afshin et al, 2017). Obesity and its related complications comprise a serious global public health problem. The pathogenesis of obesity is complex and not yet fully under-stood. Traditional treatments for obesity include lifestyle intervention (diet and exercise) and weight-loss drugs, but their weight-loss effects are not ideal.
As a new type of treatment, bariatric surgery has unique advantages in terms of weight loss, prevention of diabetes, and reduction of other cardiovascular diseases (CVDs). Due to the miraculous effect of bariatric surgery on obesity and related metabolic complications, Rubino et al. (2014) proposed changing its name from 'bariatric' to 'metabolic' surgery, and this has been widely accepted. In a joint statement of the International Diabetes Organization issued in June 2016, metabolic surgery was included in the recommended treatments for type 2 diabetes mellitus (T2DM), providing a good basis for clinical practice. However, metabolic surgery is a double-edged sword: to maximize its benefits, considerations are needed regarding the individualization and precision of the surgical style, the standardization and scientification of postoperative management, and establishment of an optimal balance between long-term effectiveness and safety.
Amounts of metabolic surgery and procedures
Laparoscopic metabolic surgery, in the form of laparoscopic vertical-banded gastroplasty, was first completed in mainland China by Professor Chengzhu Zheng of Changhai Hospital
Affiliated with the Second Military Medical University, in April 2000 (Ding and Zheng, 2016). With the maturity and development of metabolic surgery technology, growing number of hospitals are carrying out metabolic surgery therapy, which benefits continually increasing number of patients with obesity and T2DM. In the past 5 years, > 5000 patients each year in China underwent metabolic surgery (Ding and Zheng, 2016; Yang et al., 2019).
Metabolic surgery is an operation that reduces weight and improves obesity-related complications by changing the volume and order of the gastrointestinal tract. Procedures are classified as malabsorption, restricted absorption, and combined malabsorption. During the past three decades, surgeons have continuously optimized surgical procedures around long- term effectiveness and optimal surgical safety. These surgical procedures include adjustable gastric band, bowel transplantation [Roux-en-Y gastric bypass (RYGB)] , mini-reconstruction (mini-gastric bypass), sleeve gastrectomy (SG), biliary and pancreatic rejection
(biliopancreatic diversion) or duodenal displacement biliopancreatic diversion with duodenal switch), intragastric balloon dilation, and gastric electrical stimulation.
These distinct surgical procedures have substantial differences in efficacy and adverse events. The main metabolic procedures currently recommended by international and domestic guidelines are SG and RYGB, which have become more effective in treating morbid obesity. Based on Greater China Metabolic and Bariatric Surgery Database (GC-MBD), Li et al. (2021a) found that in mainland China, SG comprised 84.9% of all metabolic surgeries in 2020 and RYGB comprised 5.9% of such surgeries. SG is a simple operation, which does not change the gastrointestinal anatomical sequence and has few complications, although its long-term efficacy is relatively poor. Lazzati et al. (2020) reported that the rates of weight regain at 2 and 6 years after surgery were 5.7% and 75.6%, respectively. The proportion of patients requiring corrective surgery was 12.2% at 10years after the initial surgery in a retrospective analysis of 224718 SGs in France (Lazzati et al, 2020). In the treatment of morbid obesity, although SG has a good shortterm weight-loss effect and the percentage of excess weight loss (%EWL) can reach 36%- 85%, its long-term effect remains suboptimal, and many patients must undergo secondary corrective surgery (Shi et al, 2010). In contrast, RYGB involves a reduction in gastric volume with exclusion of 100cm of the small intestine. Its weight-loss effect is better than that of SG. However, because the pylorus is not preserved, the incidence
of postoperative dumping syndrome is 11.7%- 18.8% (Emous et al, 2017), and varying extents of postoperative malnutrition have been reported (Mangan et al., 2019). There are fewer com- plications with SG than with RYGB (Lee et al, 2015c). Globally, laparoscopic SG as a separate surgical method has become more popular than laparoscopic RYGB surgery in recent decades. However, the reappearance of weight loss and comorbidities remains problematic during long-term follow-up (Huang and Katakwar, 2020).
Many novel procedures have been developed involving SG along with some form of intestinal bypass or other alteration in small intestine anatomy. These 'sleeve plus' procedures show better technical feasbility and are associated with less postoperative morbidity (Huang and Katakwar, 2020).
Evaluation of metabolic surgery efficacy for obesity and T2DM
In this paper, we focus on SG and RYGB in China.
Weight loss
The efficacy of weight loss after an operation varies greatly among hospitals. The percentages of total weight loss (%TWLs) were 33.4%- 33.8%, 28.8%, 26.6%, 18.0%, 15%- -28.3%, and 26.6% at 1, 2, 3, 4, 5, and 10years after SG, respectively. At the same intervals, the %EWLs were 62.8%-92.2%, 49.5%-65.2%, 39.8%-65.7%, 32.2%- 53.2%, 19.5%- -80.1%, and 70.5%,respectively (Zhang et al, 2014; Liu et al., 2015; Du et al, 2016; Chang et al., 2018; Hans et al., 2018). Among patients who underwent RYGB, mean body mass index (BMI) decreased by 18.1% from 30.31 +5.38 kg/m2 to
24.45士3.79 kg/m2 at 2 years after surgery (P < 0.001) (Mazidi et al, 2017b). Furthermore, %EWLs were 76.5% and 76.2% at 3 and 5 years after surgery, respectively (Zhang et al., 2014; Du
et al., 2016). Some studies have shown that patients experienced significantly greater weight loss after RYGB than after SG, such that %TWLs were 31.0% and 27.1% (P= 0.049), %EWLs were 92.3% VS. 81.9% (P= 0.003), and changes in BMI were 11.0 kg/m2 vs. 9.1 kg/m2 (P= 0.017), respectively (Zhang et al., 2014; Yang et al., 2015; Du et al., 2016). However, other studies reported minimal differences in weight loss between the two procedures (Huang et al., 2015; Luo et al., 2020).
T2DM remission and improvement of diabetic complications
Diabetes remission is an important index for evaluating the efficacy of metabolic surgery. After SG, when T2DM remission was defined as glycated hemoglobin (HbA1c) <6.0% without the use of medication, the T2DM remission rates were 47.0%- -72.9% (Lee et al., 2011, 2020; Hans et al., 2018; Wang et al, 2019a), 57.9% (Ke et al, 2017), 78.6% (Yang et al., 2015), and 42.4% (Lee et al, 2020) at 1, 2, 3, and 5 years, respectively. When optimal glycemic control was defined as HbA1C <6. 5%, the T2DM remission rates were 47.9%, 60.0%, and 69.2% at 1, 2, and 3 years, respectively (Wang et al., 2016c). After RYGB, when T2DM remission was defined as HbA1c <6.0% without the use of medication, the T2DM remission rates were 62.8% (Yu et al, 2016d) and 28.2% (Ke et al., 2017) at 1 and 2 years, respectively. When optimal glycemic control was defined as HbA1c<6.5%, the T2DM remission rates were 70.6%- 93.0% (Lee et al, 2011; Yan et al., 2013a; Di et al, 2016; Du et al, 2018b; Wang et al, 2020a), 55.6%- 61.0% (Di et al., 2016; Du et al, 2018b; Wang et al, 2020a), and 49.2% (Wang et al., 2020a) at 1, 3, and 5 years, respectively.
In patients with obesity, the T2DM remission rate was 64.0% at 5 years after metabolic surgery, but the rates were 3.0% (Feng et al, 2019) and 2.8% (Hsu et al., 2015) at 1 and 5 years, respectively, in patients who received medical (nonsurgical) treatment. Thus, the effect was much better in patients who underwent metabolic surgery than those received medical treatment, e.g. glucagon-like peptide-1 (GLP-1) receptor agonists (Yong et al., 2012; Hsu et al., 2015; Feng et al., 2019; Wu et al., 2020b).
In some studies, the postoperative T2DM remission rate is higher after RYGB than after SG (Lee et al, 2011, 2015a), especially in older patients (Huang et al, 2015). However, most studies have shown no obvious difference between the two procedures in terms of diabetes remission (Yang et al., 2015; Du et al, 2016, 2017; Wang et al., 2019a). Yang et al. (2015) found that at 36-month follow-up. 78.6% of patients in the SG group and 85.2% of patients in the RYGB group had achieved complete remission of T2DM with HbA1c . <6.0% (P= 0.525). Surgical safety, remission of T2DM,and remission of other obesity-related comorbidities were reportedly comparable between RYGB and SG (Du et al., 2017). Other studies involving large randomized controlled trials, such as the SM-BOSS and SLEEVEPASS trials, have also confirmed these results (Schauer et al, 2017; Peterli et al., 2018; Gronroos et al., 2021).
In some studies, a lower remission rate for T2DM was observed in patients with lower preoperative BMI (27.5-32.4 kg/m) than those with higher BMI (232.5 kg/m) among patients who underwent RYGB (Ke et al., 2017; Du et al., 2018b) or SG (Wu et al., 2020a). However, 10% of patients with T2DM who had BMI < < 28.0 kg/m2 exhibited T2DM remission according to the changes in BMI and waist circumference. Fasting plasma glucose, HbA1c, and insulin were reportedly significantly improved at 1 year after RYGB liang et al., 2014; Yin et al., 2014; Wang et al., 2016b; Gong et al., 2017), implying that RYGB surgery is beneficial for patients with T2DM. In other studies, T2DM remission did not significantly differ according to the degree of obesity after RYGB (Xu et al, 2015; Zhang et al., 2017a). The reasons for the different remission rates of diabetes after SG and RYGB are due to procedure-related changes in the gastrointestinal structure, as well as the patient's degree of obesity, age, duration of diabetes, and insulin secretion, along with differences in definitions of diabetes remission.
After achievement of blood glucose control, chronic diabetes-related complications are improved or even reversed. Zhang et al. (201 5) retrospectively observed 101 patients with diabetic nephropathy (DN) stages I-IV who underwent RYGB and found that the overall remission of DN stages II and IV (microalbuminuria <30 mg/day) was 58.3% at 1 year after RYGB. Preoperative albumin/creatinine ratio and serum creatinine levels might be predictors of DN remission, and their suggested cut-off points were 1 26 mg/g creatinine and 57 μmol/L, respectively (Zhang et al, 2015). Hou et al. (2013) measured the glomerular filtration rate (GFR) in 233 patients with severe obesity before and > 12 months after bariatric surgery. At 1 year after metabolic surgery, the mean GFR decreased from 146.4+17.1 ml/min to 133.9土25.7 ml/min in the hyperfiltration group, but increased from 105.7+9.6 ml/min to 114.2土22.2 ml/min in the normal group, from 76.8士16.7 ml/min to 93.3士 20.4 ml/min in the chronic kidney disease stage 2 group, and from 49.5+6.6 ml/min to 66.8土 19.3 ml/min in the chronic kidney disease stage 3 group. Metabolic surgery- induced weight loss therefore had positive effects on renal function at 1 year after surgery (Hou et al., 2013). Chang et al. (2021) found that at the 2-year follow-up after surgery, patients with obesity or overweight who had T2DM and underwent metabolic surgery exhibited elevated GFR and reduced albuminuria. Furthermore, Wang et al. (2016a) used hyperinsulinemic-euglycemic clamps with tracer infusion in DN, DN treated with food restriction, DN treated with Roux-en-Y oesophagojejunostomy surgery (RYE), hence DN-RYE), and DN-RYEJ sham with age-matched normal rats (n = 6/group). They per- formed hematoxylin and eosin staining of kidney tissue, measured 24-h urine albumin excretion rate and GFR, and assessed the kidney messenger RNA (mRNA) content, protein content, and distribution of phospho-c-Jun NH2-terminal kinase (phospho-JNK),monocyte chemattractant protein.1 . transforming growth factor-β1 (TGF-β1), and mitogen-activated protein kinase phosphatase 5 (MKP-5) in these rats. In this DN rat model, Wang et al. (2016a) found that RYEJ surgery ameliorated renal function and attenuated glomerular hyper-trophy by improving urine albumin excretion rate and GFR.This considerable nephroprotective function may be mainly at-tributed to reduced levels of the phospho-JNK-mediated inflammatory response and increased level of the antiinflammatory mediator MKP-5 after RYEJ. The improvements in renal function and inflammation did not entirely depend on the magnitude of weight loss (Wang et al, 2016a).
Additionally, Chang et al. (2021) reported that at the 2-year follow-up after surgery, patients with obesity or overweight who had T2DM and underwent metabolic surgery demonstrated improved vibration sensation when tested with a tuning fork, compared with the medical group. Specifically, the surgical group had higher right (0.84 Vs. -0.04, crude P< 0.001) and left (0.02+0.96 vs. 0.33 +0.94, crude P= 0.016) toe tuning fork vibration scores, respectively, compared with the medical group (Chang et al., 2021). Yu et al. (201 6a) found that arterial stiffness evaluated by pulse wave velocity was significantly greater in the obesity group at baseline than in the control group (6.9+1.5 ms vs.5.8+6 ms, P< 0.01). This measure im- proved at 12 months after RYGB and was correlated with the decreased visceral fat area (VFA) resulting from this surgery in Chinese patients with obesity and T2DM (Yu et al., 2016a;Chen et al., 2017).
Metabolic syndrome (MetS) remission
MetS is a clustering of at least three of the following five medical conditions: abdominal obesity, high blood pressure, high blood sugar, high serum triglycerides, and low serum high-density lipoprotein. 'MetS remission' is determined when subjects no longer fulfill the criteria for MetS (Nora et al., 2014). Obesity is an important component and cause of MetS. Metabolic surgery is the most effective method to treat obesity and resolve the associated MetS (Lee et al, 2004; Yu et al., 2016c; Du et al., 2018a). Lee et al. (2004) prospectively observed 645 consecutive patients with morbidly obesity and found that MetS was prevalent in 52.2% of those patients. Moreover, the remission rate after laparoscopic verticalbanded gastroplasty or laparoscopic gastric bypass was 95.6% (Lee et aL., 2004). Yu et al. (2016c) reported that all MetS com- ponents improved in the RYGB group and the medication use decreased at 1 year after surgery. The prevalence of MetS, as defined by the Chinese Joint Committee for Developing Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults, decreased from 83.3% (108 patients) at baseline to 16. 5% (20 patients) at 1 year after RYGB, which was considerably better than traditional medical treatment (from 88 patients [94.6%] to 76 [81.7%] in the control group) (Yu et al., 2016c).
Using the International Diabetes Federation criteria for the diagnosis of MetS in Asian populations, Du et al. (2018a) retrospectively reviewed 97 patients who underwent laparoscopic RYGB and found a remission rate of 82.5% at 1 year after surgery. In that study, patients who underwent SG had a similar remission rate (74.7% at 1year) (Du et al., 2018a). There have not been extensive long-term follow-up studies aftermetabolic surgery (e.g. for intervals of >10 or > 20 years).MetS is an important risk predictor for CVD. Its improvement can substantially delay and reduce the occurrence of cardiovascular events and increase the life span and quality of life of patients. Metabolic surgery is beneficial for hypertension control, such that 87% of patients with hypertension who were morbidly obese before surgery experienced resolution of hypertension after surgery (Yin et al, 2019). RYGB surgery improves various parameters including cardiovascular function in patients with obesity, hypertension, and T2DM. This is presumably partially related to improvements in abnormal metabolic panel results and reductions of inflammation (Liang et al, 2013).
Fat deposits in the liver are a manifestation of MetS. Nonalcoholic fatty liver disease (NAFLD) has a global prevalence of 25% and is associated with an elevated risk of CVD. Expert consensus has proposed replacing the term NAFLD with metabolic dysfunction-associated fatty liver disease (MAFLD) to more appropriately describe the liver disease related to metabolic dysfunction. MAFLD is closely intertwined with T2DM, obesity, and dyslipidemia, all linked to an elevated risk of CVD (Dongiovanni et al., 2021). Tai et al. (2012) histologically assessed 21 patients with morbidly obesity receiving an intraoperative liver biopsy and a follow-up liver biopsy at 1 year after RYGB. Preoperatively, 19.0%, 52.4%, and 28.6% of patients had NAFLD activity scores of≥5, 3-4, and <2, respectively. All patients had NAFLD activity scores ≤2 after surgery. Fibrosis stage also showed significant improvement (P< 0.01) (Tai et al, 2012). Li et al. (2020a) found that, compared with baseline levels, the liver fat fraction evaluated by magnetic resonance imaging (a noninvasive technique) also significantly decreased at 3 months after surgery (P < 0.001).
RYGB can reduce NAFLD before its weight-loss effects become apparent, although the detailed mechanisms remain unclear. Severing the gastric vagus nerve during RYGB could re- move negative control from the central nervous system and result in increased postprandial gastric nesfatin-1 after surgery, thereby improving NAFLD (Wang et al, 2020b). Yuan et al.(2018) found that hepatic expression of Yin Yang 1 is associated with MAFLD progression in patients undergoing metabolic surgery. Yang et al. (2020) found that changes in serum nesfatin-1 after SG are associated with improvements in MAFLD.
Elevated blood uric acid is also a manifestation of MetS. Compared with baseline levels, serum uric acid levels significantly increased at 1 week after metabolic surgery (Li et al, 2021b), and then significantly decreased during long-term follow-up (Liu et al, 2019b). The mean reductions (95% confidence intervals [CI) of serum uric acid in all patients and patients with gout were 84.3 (63.1-105.4) and 163.6 (103.9-223.3) μmol/L, respectively, at 12 months after metabolic surgery. Gout may be an indicator for surgical treatment in patients with severe obesity (Lu et al, 2021). Metabolic surgery-induced weight loss and resolution of inflammatory markers, as well as changes in gut microbiota, may be responsible for the reduced serum uric acid level and xanthine oxidase activity (Lu et al, 2020).
Zhao et al. (2017c) found that RYGB surgery is an effective treatment to reduce 10-year cardiovascular risk according to the United Kingdom Prospective Diabetes Study Risk Engine in Chinese patients with diabetes who had obesity. Waist-to-hip ratio, age, low-density lipoprotein cholesterol, and HbA1c were the most important factors influencing coronary heart disease and stroke risks after RYGB surgery (Zhao et al., 2017c). Mazidi et al. (2017a) found that changes in adiposity and other cardiometabolic risk factors improved at 12 months after RYGB in Chinese patients, such that 10-year and fatal coronary heart disease risks decreased from 8.8% to 4.6% and from 4.6% to 2.1 %, respectively (both P < 0.001), and CVD risk decreased by 50%, at 1 year after surgery. These findings confirmed the efficacy of metabolic surgery for T2DM treatment. Compared with SG, gastric bypass surgery has more power to reduce CVD risk (Wei et al, 2018a). Hung et al. (2021) found that patients had a significantly lower risk of cardiovascular events after meta-
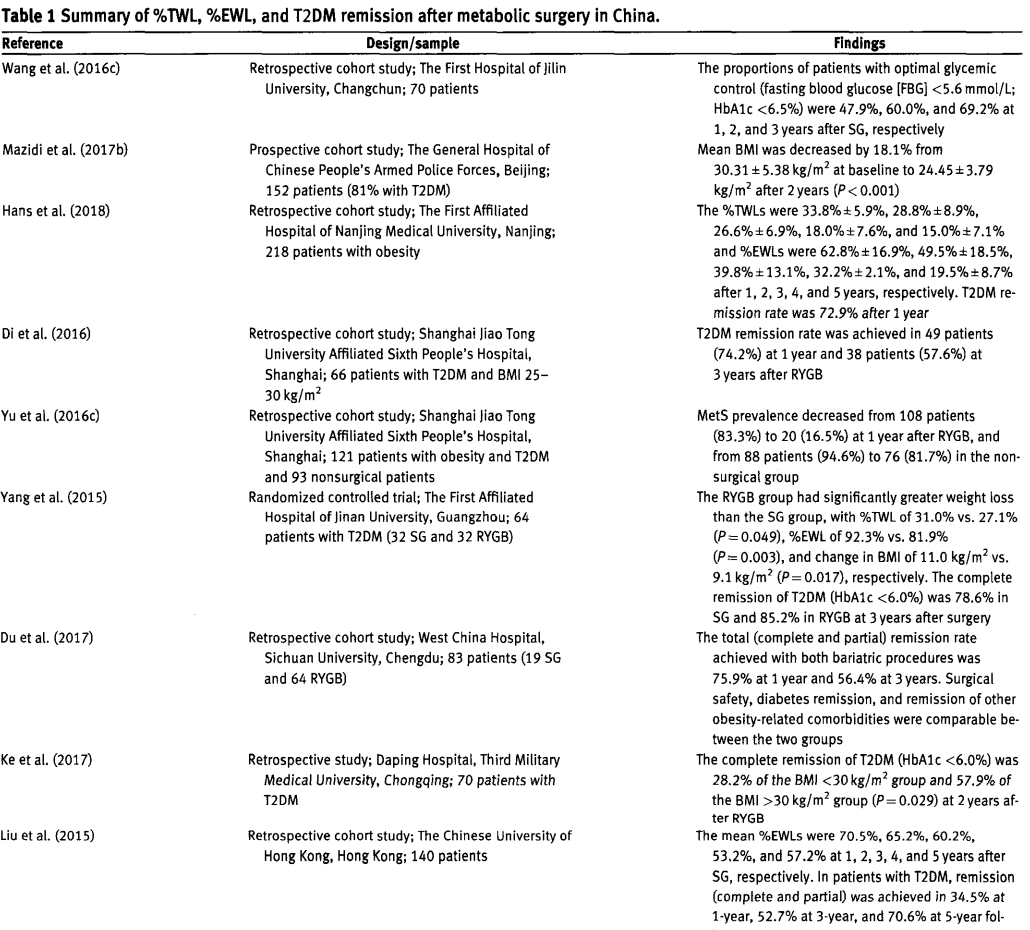
bolic surgery (hazard ratio = 0.168; 95% CI = 0.085 - 0.328; P< 0.001) than patients in the nonsurgical group, such that the CVD risk after metabolic surgery was not significantly different from that in general population (hazard ratio = 1.202; 95% CI= 0.585- 2.471; P= 0.617). Among patients hospitalized with heart failure, prior metabolic surgery has been associated with better in-hospital outcomes, mainly in patients who had successful weight loss (Han et al., 2019b). Among hospitalized patients with acute ischemic stroke in the Nationwide Inpatient Sample, prior metabolic surgery with BMI <35 kg/m2 was associated with lower in-hospital mortality, fewer poststroke complications,improved disability status, and better healthcare utilization (Han et al, 2019a). Wong et al. (2021) estimated the risks of postbariatric severe hypoglycemia, CVD, end-stage kidney diseases, and all-cause mortality in patients with obesity and T2DM. Over a mean follow-up period of 3 months covering 5725 person-years, compared with nonsurgical patients, the surgical group had a significant reduction in the risk of CVD events (hazard ratio = 0.464, P=0.015) and no occurrence of mortality events (Wong et al, 2021). The %TWL, %EWL, change in BMI, and diabetes remission findings after metabolic surgery in China are summarized in Table 1.

Other obesity-related complications
Metabolic surgery has a strong and durable metabolismimproving effect. In addition to the relief of diabetes and its complications, as well as relief of adverse blood pressure and blood lipids, this surgery is beneficial for other obesity-related complications.
Obstructive sleep apnea (OSA)-hypopnea syndrome is highly prevalent (82.2%) in patients with obesity (Yeh et al.,2010). Zou et al. (2015) used the polysomnography test on 72 . consecutive patients with obesity and T2DM before and at least 6 months after RYGB. They found that the prevalence of 0SAhypopnea syndrome was 75.0%, with a mean preoperative apnea-hypopnea index (AHI) of 22.4 events/h (95% CI=17.0-27.9) at baseline and 7.1 events/h (95% CI=4.2- 9.9) at the second visit. Preoperative polysomnography tests showed that 22, 9,and 13 patients had mild (5≤AHI≤15 events/h),moderate (15 < AHI≤30 events/h), and severe (AHI > 30 events/h) OSA, respectively. The follow up period revealed that 28 (63.6%) patients were cured of OSA and 35 (79.5%) patients showed substantial improvement. Thus, RYGB can be an effective therapeutic intervention in the management of 0SA-hypopnea syndrome for patients with both obesity and T2DM, and the preoperative AHI| and age might be important factors that influence its effect (Zou et al., 2015).
Xu et al. (2016) assessed 35 consecutive patients with OSA who underwent RYGB and found significant improvements in the following sleep parameters in both men and women at 6-24 months after surgery: Epworth Sleepiness Scale, AHI, mean oxygen saturation (%), lowest pulse oxygen saturation (%), and oxygen desaturation index. Epworth . Sleepiness Scale decreased from 7.7 (5.5-10.7) and 6.5 (4.8-8.5) at baseline to 3.4 (2.3-5.3) and 3.0 (2.0-4.4) after surgery in men and women, respectively; AHI decreased from 21.7 (15.9-30.3) and 21.3 (15.4-28.3) to 6.2 (3.4-15.3) and 8.9 (5.3-15.2), respectively; mean oxygen saturation increased from 93.6% (92.1%- 94.5%) and 94.0% (93.1%- 94.8%) to 95.4% (94.3%-96.4%) and 95.7% (95.1%- 96.2%) respectively; lowest pulse oxygen saturation increased from 76.1%
(69.6%-81.1%) and 77.8% (72.1%-82.0%) to 87.5% (82.4% - 89.6%) and 85.9% (82.6%- 88.7%), respectively; and oxygen desaturation index decreased from 23.9 (16.8-33.8) and 28.5 (20.8-38.3) to 4.6 (2.4-12.5) and 8.7 (5.1-15.0), respectively Xu et al., 2016). Moreover, higher baseline BMI has been linked with greater reduction of AHI after metabolic surgery (Li et al., 2015).
Importantly, obesity is dominated by obstructive pulmonary ventilation dysfunction, and treatment of MetS provides an improvement in lung function. Tu et al. (2015) retrospectively reviewed 32 patients with obesity and T2DM before and 6 months after RYGB. They found that forced expiratory volume during 1 sec increased from 2.78 L to 2.97 L, percentage of forced expiratory volume during 1 sec increased from 91.6% to 97.7%, forced vital capacity increased from 3.22 L to 3.39L, and percentage of forced vital capacity increased from 87.7% to 92.0%. Thus, all of these parameters had significantly im. proved at 6 months after surgery, and the increases were negatively correlated with decreases in body weight and VFA (Tu et al, 2015). Among patients with SG, Ruze et al. (2020) found that the improvement of lung function after surgery is related to improvements in alveolar structures, surface protein expression induced by weight loss, and enhanced glucose metabolism. Furthermore, compared with a nonsurgery group, the metabolic surgery group was at significantly lower risk of respiratory tract infections among patients with obesity (adjusted hazard ratio= 0.432,95% CI= 0.340- 0.549,P< 0.001) (Chen et al., 2021).
Obesity is recognized as a risk factor of gastroesophageal reflux disease, which is pervasive among Chinese patients with morbid obesity. RYGB substantially improves reflux symptoms and erosive esophagitis (Tai et al, 2009). Tai et al. (2009) evaluated 150 Chinese patients with morbidly obesity who underwent RYGB, as well as 300 age- and sex-matched controls, and found that patients with morbid obesity had higher frequencies of reflux symptoms (16% VS. 8%, P= 0.01) and erosive esophagitis (34% Vs.17%,P<0.01). In addition to substantial weight loss, the prevalences of reflux symptoms and erosive esophagitis decreased significantly after surgery (19.2% Vs. 0%, P= 0.05, and 42.3% Vs. 3.8%, P< 0.01, respectively) (Tai et al, 2009).
Obesity can be accompanied by changes in endocrine hormones, including thyroid-related hormones (e.g. reduced male androgen levels). Total testosterone levels substantially increase in Chinese men with obesity and T2DM after RYGB (Liu et al., 2018a) and SG (Zhu et al, 2019c). This occurs partly through reductions in adipose tissue, especially visceral fat (Liu et al., 2018a), as well as substantial weight loss, serum uric acid reduction, insulin resistance (R) improvement (Zhu et al, 2019c), and amelioration of inflammation after SG (Zhu et al., 201 9b). Furthermore, metabolic surgery promotes erectile function (Kun et al, 2015; Liu et al., 2020). Patients with obesity who undergo metabolic surgery have higher chances of pregnancy, successful delivery, vaginal delivery, and fewer maternal complications during labor. Metabolic surgery also
increases the probability of pregnancy in patients with obesity (Hsieh et al., 2020).
Notably, there have been reports of changes in serum thyroid hormone levels after metabolic surgery. Mean free thyroxine levels decreased from 16.26 pmol/L to 14.59 pmol/L (P < 0.01) and thyroid-stimulating hormone (TSH) levels decreased significantly (2.19士1.09 mIU/L vs. 1 97士1.12 mlU/L, P= 0.027) at 6 months after RYGB (Liu et al., 2017a). In other studies, thyroid hormones including free triodothyronine, free thyroxine, and TSH declined in parallel at 12 months after RYGB and SG (Yu et al, 2019; Zhu et al, 2019a). However, weight loss after SG is accompanied by a significant decrease in serum TSH level, but no change in serum free thyroxine or free triodothyronine levels (Yang et al, 2017). Decreased TSH levels after weight loss induced by RYGB might be mediated by a decline in leptin (Yu et al, 2019). However,patients after RYGB have a potential risk of thyroid nodule progression (Zhang et al, 2017b). Prospective studies with large samples are needed to further confirm the changes in endocrine hormones after metabolic surgery.
Obesity is a risk factor for physical morbidities and negatively influences psychosocial functioning and the capacity to live a full and active life. To identify the effect of bariatric surgery on psychosocial functioning in Chinese patients, Wei et al. (2020) conducted a multicentre, prospective, observational cohort study in Hong Kong involving 25 patients who underwent bariatric surgery (SG: 96%; RYGB: 4%) and 25 control patients matched using propensity scores derived from baseline covariates. The Short-Form-12 Health Survey Version 2, EuroQOL 5-di- mension-5-level, and lmpact of Weight on Quality of Life-Lite measures were used to assess health-related quality of life at follow-up interviews via telephone at 1, 3, 6, and 12 months after surgery. Scores of anxiety and depression were evaluated using the Hospital Anxiety and Depression Scale. At 12 months after metabolic surgery, an increase in physical quality of life, a reduction in depression status, and less weight-related impairment were observed (Wei et al, 2020). Wu et al. (2016) recruited 60 Chinese patients with obesity who underwent laparoscopic adjustable gastric banding or laparoscopic SG. Patients were invited to complete the Chinese Hong Kong Medical Outcomes Study Short-Form Health Survey at baseline and 1 year after surgery. Mean absolute weight reduction at 1 year after surgery was 19.8 kg. Metabolic surgery resulted in significant gains in survey scores in all physical domains and in three of the four psychological domains. Such surgery offers greater health-related quality of life improvements, as well as significant reductions in body weight, in patients with prediabetes and diabetes, compared with normoglycemic individuals (Wu et al, 2016).
Predictors
Predictors of weight loss
Researchers are investigating predictors of metabolic surgery. %EWL is the most commonly used outcome metric in the bariatric surgery.related literature. It reports the percentage of weight loss relative to an 'ideal' BMI, and numerous studies have arbitrarily defined suboptimal weight loss as %EWL <50%. However, recently published studies have suggested that %TWL yields a more accurate reflection of outcomes with out the need to define an ideal body weight (Van De Laar, 2012; Hatoum and Kaplan, 2013). Tu et al. (2021) retrospectively investigated 430 patients with obesity who underwent metabolic surgery and found that 397 patients (92.3%) exhibited MetS at baseline and 337 patients (84 .9%) were in MetS remission 1 year after surgery. Multivariate regression analysis indicated that, compared with %EWL, %TWL better predicts MetS remission at 1 year after metabolic surgery (P for trend = 0.029). Furthermore, Youden's index indicated that ≥25%TWL is the optimal metric to identify individuals who are good responders to bariatric surgery (Tu et al., 2021). Mao et al. (2021) were the first to develop a predictive nomogram for the effect of weight loss based on the clinicopathologic data of 380 patients with obesity who underwent
laparoscopic RYGB, SG plus jejunal bypass, or laparoscopic SG. The predictors included age, sex, surgical procedure, hyperlipidemia, blood pressure, hyperuricemia, BMI, and waist circumference. The proposed nomogram resulted in more accurate nonremission prediction for patients with obesity after metabolic surgery and may provide a reference for the preoperative choice of surgical procedures (Mao et al,2021). With successful weight loss defined as %EWL > 50%, a %EWL value of 56. 54% at 6 months was the best predictor of successful weight loss at 5 years (sensitivity = 72.38%,specificity = 82.69%) in Chinese patients with morbidly obesity (Wang et al., 2021).
Predictors of T2DM
Although various predictive factors for diabetes remission after metabolic surgery have been reported, many preoperative clinical factors with the potential to predict T2DM remission have great similarities.
T2DM remission has been negatively associated with age, diabetes duration, insulin use, and HbA1C levels. BMI and C-peptide levels have been positively associated with the remission rate in Chinese patients. However, no significant association has been reported between sex and remission rate. Multiple regression analysis has shown that greater waist circumference, lower fasting plasma glucose, and higher fecal calprotectin at 2 h in an oral glucose tolerance test are independently associated with diabetes remission at 1 year after surgery (Du et al., 2018b). Yu et al. (2015a) reported that shorter duration of diabetes, lower preoperative HbA1c level, and higher fasting C-peptide level were predictive of short-term diabetes remission after RYGB surgery in Chinese patients, as was greater VFA. This VFA finding was cited by the Joint Statement by International Diabetes Organizations in 2016 (Rubino et al., 2017) and the Standards of Medical Care in Diabetes by the American Diabetes Association in 2018- 2021 (American Diabetes Association, 2018,2019, 2020, 2021). Ke et al. (2021) found that visceral adiposity index, HbA1C, and duration of diabetes can predict diabetes remission after metabolic surgery and that an index of 4.46 is a useful threshold for predicting surgical efficacy. Preoperative fasting plasma C-peptide levels are a predictor of remission of T2DM after metabolic surgery (Lee et al, 2012; Zhao et al., 2018).
Thus far, >10 models have been developed to predict T2DM remission after metabolic surgery. The ABCD, DiaRem, advanced-DiaRem, and DiaBetter scores are among the most commonly used models. Lee et al. (2015b) proposed the ABCD diabetes surgery score (a multidimensional grading system composed of age, BMI, C-peptide levels, and T2DM duration) to predict success after metabolic surgery in T2DM treatment. The DiaRem score includes the factors of age, HbA1c, medication, and insulin usage. The ABCD score is better at predicting T2DM remission at 1 year after surgery, compared with the DiaRem score, following RYGB (Lee et al.,2016) or SG (Lee et al., 2015b). However, Kam et al. (2020) compared the ABCD,DiaRem, advanced-DiaRem, and DiaBetter scores by the area under the receiver operating characteristic curve, the calibration evaluated by HosmerLemeshow goodness-of-fit tests and predicted-to-observed ratios in a total of 253 RYGB patients (214 and 131 completed 1- and 3-year follow-ups, respectively), and found that the DiaBetter model (including HbA1c levels, T2DM duration, and antidiabetic medication used) was the optimal model for predicting postoperative diabetes remission in Chinese patients with diabetes. Regarding predictors of MetS remission, multiple logistic regression analyses indicated that a shorter diabetes duration and higher %EWL were associated with a greater chance of MetS remission after RYGB surgery (Yu et al., 2016c).
Mechanism for weight loss and T2DM remission
The weight loss and hypoglycemic mechanisms of metabolic surgery are extremely complicated, which involve food restriction and/or malabsorption, changes in gut microbiota, bile acid metabolism, and gastrointestinal hormones.
Gut microbiota
Metabolic surgery substantially changes the model of the gut microbiota.
Liu et al. (2018b) randomized diabetic rats to RYGB or sham surgery, and then collected stool samples at baseline and at 8 weeks after surgery. The samples were analyzed using 16S ribosomal RNA gene sequencing. The RYGB group showed enrichment of Bacteroidetes, Proteobacteria, Fusobacteria, and Actinobacteria, whereas the sham surgery group showed enrichment of Firmicutes and Verrucomicrobia. Additionally, Liu et al. (2018b) performed a case-control study of the gut microbial community profiles of patients with T2DM, compared with the profiles of healthy individuals, via gene sequencing of mucosal-luminal interface samples collected from the ascending colon during colonoscopy. They found that the family Coriobacteriaceae within Actinobacteria might contribute to the beneficial effects of RYGB on T2DM (Liu et al, 2018b).Furthermore, Liu et al. (2017b) found that Bacteroides thetaiotaomicron, a glutamate-fermenting commensal, was markedly decreased in individuals with obesity and was inversely correlated with serum glutamate concentration through metagenomic sequencing. Nie et al. (2020) found that within the species of gut microbiome negatively correlated with abdominal VFA, Eubacterium eligens had the strongest correlation. Functional analyses showed that among all the obese parameters, VFA had the strongest correlation coefficients with the obesity-related microbial pathways. Microbial pathways involved in carbohydrate fermentation and biosynthesis of L-glutamate and L- glutamine might contribute to visceral fat accumulation (Nie et al, 2020).
Zhong et al. (2016) assigned diabetic rats to two groups to receive duodenojejunal bypass (DJB) or a sham operation. When the DJB was completed, diabetes recurrence was induced. The DJB rats were then grouped by blood glucose level into the DJB-remission (DJB-RM) and DJB-recurrence (DJB-RC) groups. Zhong et al. (2016) found that the relative abundances of Firmicutes were higher in the control (58.06% 土11.12%; P< 0.05 VS. sham; P< 0.05 VS. DJB-RC) and DJB-RM (55. 58%+6.16%; P<0.05 Vs. sham; P<0.05 VS. DJB-RCgroups than in the sham (29.04%士 1.36%) and DJB-RC groups than in the sham (29.04%士 1.36%) and DJB-RC (27.44%士土2.17%) groups. However, the relative abundances of Bacteroidetes were lower in the control (33. 46%土10.52%; P<0.05 vs. sham; P<0.05 VS. DIB-RC) and DJB-RM (34.63%+3.37%; P<0.05 Vs. sham; P<0.05 vs. DJB-RC) groups than in the sham (46.88% 士2.34%) and DJB-RC (47.41%士5.67%) groups. Compared with the control group, the abundance of Firmicutes was significantly higher in rats after DJB, and the abundance of Bacteroidetes was lower in those rats (Zhong et al., 2016). The gut microbiota was reportedly altered by DJB, producing more Firmicutes and Proteobacteria but less Bacteroidetes in a diabetic rat model (Zhang et al., 2016). Shao et al. (2017) found that RYGB, but not SG, altered the gut microbiota of Sprague-Dawley rats. RYGB also reduced the diversity of gut microbiota. Furthermore, the abundance of gamma proteobacteria was negatively correlated with postoperative body weight and may contribute to stable weight loss after metabolic surgery (Shao et al, 2017). Moreover, SG reshapes the diversity of the gut microbiota and improves its diurnal oscillation, thus affecting host metabolism (Shao et al., 2018). Several studies have shown that the gut microbiota of patients or animal models with IR has some unique components and structural characteristics. Floral diversity is enriched by metabolic surgery, which strongly promotes remodeling of the gut microbiota (Guo et al., 2017).
Bile acids
Bile acids have been suggested as key mediators of improvements in glucose metabolism after metabolic surgery. The mechanism may be the activation of nuclear receptors and cell membrane receptors, such as farnesoid X receptor (FXR) and the G-protein-coupled bile acid receptor TGR5, to alter the expression of numerous genes encoding enzymes/proteins involved in the regulation of glucose, fatty acid, and lipoprotein synthesis,metabolism, transport, and energy metabolism (Hylemon et al., 2009; Staels and Fonseca, 2009).
Yu et al. (2015b) found that a higher level of chenodeoxycholic acid relative to total bile acid and a shorter duration of diabetes at baseline were associated with a greater probability of diabetes remission after RYGB. In a cross-sectional study, the level of chenodeoxycholic acid relative to total bile acid was significantly higher in individuals with obesity and T2DM than among individuals with normal glucose tolerance (Yu et al., 2015b). Zhao et al. (2017a) found that the baseline stearic acid/ palmitic acid ratio was associated with the probabillity of diabetes remission after RYGB and may serve as a diagnostic marker in preoperative patient assessment.
Additionally, the circulating bile acid level increased after metabolic surgery in diabetic rats (Chen et al., 2020a). DJB preferentially increases serum taurine-conjugated bile acids, presumably because more bile acid reabsorption occurs in the terminal ileum (Zhong et al., 2016). Several studies in mice have established the role of enhanced bile acid signaling through TGR5 and FXR in metabolic improvements. However, the subtypes of bile acids and the tissue-specific signaling pathways involved remain unclear. The Bas-FXR- histone acetyltransferase steroid receptor coactivator-1 axismediated restoration of the transient receptor potential ankyrin 1 expression plays a critical role in the enhanced glucose-stimulated insulin secretion and remission of diabetes in spontaneous diabetic Goto-Kakizaki rats after RYGB (Kong et al, 2019).
Gastrointestinal hormones and cytokines
SG significantly improved glucose homeostasis, with lower ghrelin levels and higher postprandial GLP-1, peptide YY, and insulin^ levels in Goto-Kakizaki rats (Li et al., 2009; Sun et al, 2014). Fasting GLP-1 and peptide YY significantly increased in both groups, but more after RYGB. Glucagon levels significantly decreased at 1, 3, and 6 months after surgery in both SG and RYGB groups, but returned to baseline at 12 months (Yang et al, 2018). Zhang et al. (2020) found the increase of serum adiponectin level and the decrease of leptin resistance after RYGB restored the metabolic balance of leptin and adiponectin and improved IR. However, Wang et al. (2020d) found no change in fasting ghrelin at <12 months and increased fasting ghrelin at 2 24 months after RYGB.
RYGB may improve β-cell apoptosis with ghrelin overexpression in patients with BMI 32.5 kg/m2 (Yang et al, 2014) and decrease pancreatic β-cell degranulation and dediferentiation in diabetic rats (Qian et al., 2014).
Obesity has been widely recognized as a chronic inflammatory condition. Chen et al. (2009) found that elevated inflammatory indicators including C.reactive protein and white blood cell count decreased rapidly after metabolic surgery. In rat models, RYGB could efficiently restore abnormal gut permeability and reduce inflammation in the intestine, depending on reactivation of intestinal NOD-like receptor family pyrin domain containing 6 (Wang et al., 2020c). Luo et al. (2016) measured the serum metabolic profiles of 35 patients with T2DM before and after RYGB and found that improvements in insulin sensitivity (IS), energy metabolism, and inflammation were related to meta bolic alterations of free fatty acids, acylcarnitines, amino acids, bile acids, and lipids. Zhan et al. (2017) found that the follicular helper T (Tfh) cells after RYGB presented lower inflammatory status and secreted higher level of interleukin-10 (IL-10), through which these Tfh cells promoted the development of regulatory B cells. Dai et al. (2017) found that B cells after RYGB presented significantly elevated frequency of IL-10- producing cells and reduced frequency of IL-6-producing cells compared to those before RYGB. B cells before RYGB supported IL-17 secretion from T cells whereas these cells after RYGB lost such capacity. B cells after RYGB also gained the capacity to suppress T cell interferon γ production through TGF-β-mediated effects (Dai et al., 2017).
Metabolic surgery resulted in significant and distinct changes in multiple gastrointestinal and pancreatic peptide hormones that are important regulators of obesity and metabolism.
Others
In addition to gut microbiota, bile acids, gastrointestinal hormones and cytokines, metabolic surgery activates other mechanisms, such as autophagy, DNA methylation (Liu et al., 2019a), changes in microRNA expression (Qian et al., 2014; Zhu et al., 2017), changes in genetics (Li et al., 2019), endoplasmic retic- ulum (ER) stress (Li et al., 2016), changes in Na+-coupled glucose transporters (SGLT) and glucose transporter facilitators (GLUT) in the gut (Li et al., 2012; Yan et al., 2013b; Xia et al., 2019), and activating insulin signaling in the brain (Li et al., 2020b).
The hyperinsulinemic-euglycemic clamp is the gold standard test for IS. After metabolic surgery, IS improves. The glucose disposal rate significantly increased at 3 months after RYGB (from 3.36+1.26 mg/kg/min to 6.30土 1.3 mg/kg/min, P< 0.001). In clinical practice, the homeostatic model assessment index is more practical and therefore more commonly used. The value according to the homeostatic model assessment for IR reduced markedly (from 4.47士2.20 to 2.10土0.75, P< 0.001). Peripheral and hepatic IS improved remarkably at 3 months following RYGB, an important mechanism for early improvement in patients with T2DM who have low BMI (Zhao et al, 2017b). There was more fat mass loss (31.03%) in the android region than in any other body region tested. Preoperative android fat mass was significantly correlated with IR (r= 0.49, P< 0.05). Body composition in Chinese patients with T2DM is rebalanced after RYGB, and the reduction of central obesity after metabolic surgery can result in IR improvement (Li et al.,
2014).
Renal gluconeogenesis was downregulated in the rat model following RYGB surgery (Wen et al., 2015). Yan et al. (2016) divided 20 adult male rats with T2DM into sham and RYGB groups. Intestinal sections were stained with hematoxylin and eosin for light microscopy examination. The mRNA and protein expression levels of key regulatory enzymes of gluconeogenesis were determined through reverse-transcription polymerase chain reaction and western blotting, respectively. The reduced hepatic gluconeogenesis and increased intestinal gluconeogenesis may contribute to improved glucose homeostasis after RYGB in a T2DM rat model (Yan et al., 2016).
In an animal experiment, He et al. (2014) divided SpragueDawley rats into four groups, diabetic RYGB (n = 18), diabetic RYGB sham (n= 6), diabetic (n= 6), and nondiabetic control (n= 6). The diabetic RYGB group had significant decreases in weight, fat mass, and food intake. At 2 weeks after surgery, the RYGB group had significantly improved hepatic IS index by hyperinsulinemic-euglycemic clamps with tracer infusion postoperatively. Significantly increased IS and decreased lipid content in muscle were not detected until 4 weeks after Surgery. They concluded that RYGB increases hepatic and periph. eral IR through reduced lipid content of hepatocytes and skeletal muscle cells in rats with T2DM (He et al, 2014). Hepatic IS was ameliorated because of reduced hepatic lipid accumulation by upregulating shot-term hepatic autophagy after RYGB surgery in type 2 diabetic rats (He et al., 2013, 2015).
A significant change was observed in the peripheral blood microRNA expression profile of T2DM patients after RYGB surgery compared with those before operation (Zhu et al, 2017). microRNA-200a,a Rheb-targeting miRNA, regulates Rhebmediated amelioration of hepatic IR in a rat model of DJB (Guo et al., 2016).
The transcriptome sequencing results showed that the genes differentially expressed in the RYGB- and sham-operated groups were significantly enriched in the gene ontology biological processes associated with fatty acid metabolism and lipid transport. RYGB could change DNA methylation and gene expression profiles in the proximal jejunum in T2DM rats (Liu et al, 2019a).
DJB reduced hepatic ER stress and the JNK activity and induced significant improvements in glucose homeostasis and IS, but without weight loss in a diabetic rat model (Li et al., 2016).
DJB surgery reduced intestinal glucose absorption by reducing the activity and expression of SGLT1, which represents a potential therapeutic target for patients with diabetes Yan et al, 2013b). RYGB may improve IR and treat T2DM through downregulation of tumor necrosis factor a mRNA transcription, upregulation of peroxisome proliferator-activated receptor γ2, phosphatidylinositol-3-kinase subunit p85a, GLUT4 mRNA transcripts, and inducing translocation of GLUT4 in adipose tissue (Li et al, 2012). Residual gastric dilatation interferes with metabolic improvements following SG by upregulating the expression of SGLT1 (Xia et al, 2019).
RYGB induces a higher resting energy expenditure level in diabetic rats (Chen et al, 2020a). DJB ameliorates T2DM by activating insulin signaling and improving glucose utilization in the brain (Li et al, 2020b). Li et al. (2019) found that 8.4% of obesity cases were caused by change in genetics, and mutation carriers had negative effects on the efficacy of bariatric surgery.
The mechanisms of metabolic surgery in losing weight and improving metabolism require further study. And elucidation of these mechanisms could help to identify new drug targets, develop functional food, and improve surgical techniques.
Adverse events and side effects
Surgical complications
Metabolic surgery is a surgical operation and therefore has surgery-related complications. Major adverse reactions reported in the literature are rare. Hans et al. (2018) reported that among the long-term outcomes of laparoscopic SG, the most common early complication Was port site dehiscence (10%),which could be managed conservatively. Du et al. (2018b) reported that major complications included incomplete intestinal obstructions (1 .9%) and anastomotic leak (1 .0%). Importantly, rare diseases have occurred after metabolic surgery, such as fatal fulminant pancreatitis after RYGB (Wang et al., 2008) and cardia gastric cancer (Chen et al., 2020b). The relationship between metabolic surgery and these diseases requires more investigation in the future to confirm its safety.
Long-term complications
Long-term complications include hypoglycemia and dumping syndrome, malnutrition, bone fractures, and mental and psychological problems.
Osteoporosis. Lin et al. (2020) found that in patients with obesity before metabolic surgery, vitamin D deficiency [25(OH)D < 20 ng/m] and insufficiency [20 < 25(0H)D < 30 ng/mI] had prevalence of
73% and 22%, respectively. Secondary hyperparathyroidism (parathyroid hormone≥65 pg/ml) was present in 24% and hy- pocalcemia (ionized Ca <4.5 mg/d) was present in 50% (Lin et al, 2020).
Surgically induced weight loss is associated with modest reductions in lean mass, bone mineral content, and bone mineral density. Assessment of bone loss is indicated for patients undergoing metabolic surgery (Maghrabi et al., 2015). However, one trial found that the rate of peripheral fractures was similar in patients with metabolic surgery and with intensive medical therapy (Maghrabi et al, 2015). Fracture risk dif- fers among types of metabolic surgery (Zhang et al., 2018). There is a tendency toward an increased fracture risk in patients with mixed restrictive and malabsorptive procedures (risk ratio= 1.54, 95% CI= 0.96- 2.46), compared with those with restrictive procedures alone (Zhang et al, 2018). These findings suggest that RYGB may have important adverse effects on the skeleton.
Metabolic surgery may reduce calcium absorption and substantially decrease bone mineral density. Wei et al. (2018b) found that the prevalence of secondary hyperpara- thyroidism is high in patients with morbidly obesity before metabolic surgery, which is related to vitamin D deficiency. The prevalence of secondary hyperparathyroidism increased gradually after surgery. Supplementation of vitamin D and calcium must be in higher dose for patients undergoing by pass procedures, especially those involving malabsorption (Wei et al, 2018b).
Malnutrition. Guan et al. (2018) reported that nutritional deficiencies were common in Chinese bariatric surgery candidates, such that vitamin D deficiency was most widespread (78.8%). This was followed by vitamin B1 (39.2%), vitamin B6 (28.0%), folate (26.8%), vitamin C (18.0%), albumin (1 3.4%), transferrin (11.6%), and phosphorus (11.5%). Despite the routine use of postoperative multivitamin and calcium
supplements, nutritional deficiencies were evident among patients undergoing RYGB or SG (Guan et al, 2018).
lron deficiency and iron deficiency anemia are extremely frequent after RYGB in Chinese patients with obesity and T2DM. Yu et al. (2016b) found that, after RYGB, the proportion of men with anemia increased from 6.0% at baseline to 15.2% and 17.0% at 6 and 12 months, respectively, and then decreased to 4.5% at the 24-month visit. Among postmenopausal women, the proportion with anemia increased to 34.0% at the 6-month follow-up, and then gradually decreased to 25.0% and 26.7% at the 12- and 24-month visits, respectively. Among premeno-pausal women, the proportion with anemia increased dramati cally to 62.5% at the 24-month follow-up (Yu et al., 2016b). Early iron supplementation is important, especially for women of childbearing age.
Hypoglycemia and dumping syndrome. Dumping syndrome is a serious complication that is likely to become more common because of the increasing number of patients undergoing meta- bolic surgery. Sun et al. (2019) reported that mild-to-moderate symptoms suggestive of hypoglycemia and early dumping syndrome were common in patients after SG, such that prevalence rates were 66.39% (81/122) and 40.98% (50/122), respectively. Patients with symptoms suggestive of both early dumping syndrome and hypoglycemia comprised 33.61% (41/122) of the patients (Sun et al., 2019). Yu et al. (201 6d) performed continuous glucose monitoring over a 3-day period on 43 Chinese patients with obesity and T2DM, combined with a mixed-meal test before and 1 year after RYGB. The normal range of mean amplitude of glucose excur-
sions was <3.9 mmol/L,and complete diabetes remission (HbA1c <6.0%) was achieved in 27 patients (62.8%) at 1 year after RYGB. However, the mean amplitude of glucose excursions did not change after surgery, and only 18.6% patients had dual-remission' (Yu et al., 2016d). Wang et al. (2013) found that acarbose can be an effective treatment for severe hypoglycemia due to dumping syndrome.
Others. Some patients have mental and nervous system effects after metabolic surgery, such as mild or severe depression. Lu et al. (2018) explored the association between metabolic surgery and major depressive disorder in a 1 2-year nationwide Cohort study. Metabolic surgery was significantly associated with a 70% increase in the risk of major depressive disorder, mainly with malabsorptive procedures and > 4 years after surgery, compared with the risk in matched controls. Long-term malabsorption might be related to the incidence of major depressive disorder after metabolic surgery (Lu et al., 2018). Rare adverse
reactions include Wernicke encephalopathy with concurrent polyradiculoneuropathy (Chang et al, 2019).
Predictive factors for relapse of hyperglycemia
Wang et al. (2019b) reported that 54.2% of patients experienced a relapse of hyperglycemia at the 5-year follow-up after RYGB. The 1-, 3, and 5-year relapse rates were 4.2%, 12.5%, and 50.0%, respectively. The preoperative HbA1c and C-peptide levels at surgery are important for predicting the relapse of hyperglycemia after laparoscopic RYGB surgery. Lifestyle intervention reduced the hyperglycemia relapse rate (66.7% vs. 41.7%), implying that such intervention is crucial for these patients (Wang et al., 2019b). Nautiyal et al. (2020) reported that after RYGB or loop DJB with SG, 16.8% of patients had early relapse and 10.6% did not have remission. Diabetes duration is the most probable, independent preoperative predictor of early T2DM relapse or lack of remission in patients with obesity and diabetes undergoing metabolic surgery (Nautiyal et al, 2020).
Frequent relapses and significantly decreased remission rates begin after 10 and 15 years, respectively, in surgically treated patients. Concern is warranted for predictors related to gastrointestinal hormones, the metabolic profile of long-term remission, and relapse of T2DM following metabolic surgery in patients with obesity (Chen and Kong, 2018). Poor blood glucose control and poor pancreatic islet function before surgery predict the recurrence of diabetes after surgery. Health economics Healthcare costs associated with diabetes in China have considerably increased from 2.2 billion Chinese yuan in 1993 to 200 billion yuan in 2007 and are forecast to exceed 360 billion yuan by 2030 without adjustment for inflation (Wang et al, 2009). Tu et al. (2019) investigated 106 patients with obesity and T2DM who underwent RYGB and 106 patients with T2DM who received conventional medical management from three academic medical centers. They found that, compared with conventional medical management, RYGB costs $19359 (125836 yuan) per quality-adjusted life year gained (incremental costutility ratio), which is lower than the willingness-to-pay of $20277 per quality-adjusted life year. RYGB is therefore costeffective for Chinese patients with T2DM and obesity at 4 years after surgery (Tu et al., 2019). Two other studies also support that, compared with drug treatment of patients with obesity and T2DM, metabolic surgery can bring long-term economic savings (Tang et al., 2016; Wan et al., 2019), with more savings from SG (Tang et al, 2016). Wu et al. (2021) found that metabolic surgery in patients with obesity and T2DM is expensive, but leads to an improved comorbidity profile, and reduced length of hospitalization.
Conclusion
The medical consensus does not question the advantages of metabolic surgery in weight loss or improvement of hyper- glycemia and obesity-related complications. However, this surgery has various complications. Recently, there have been technological breakthroughs in the field of metabolic surgery, such as robotic surgery and endoscopic techniques. In 2018, the International Federation of Obesity and Metabolic Surgery issued a statement, proposing single anastomosis duodeno-ileal bypass with SG as a new and improved metabolic surgery. This has shown great clinical potential, but its long-term efficacy and safety remain unclear. The risk of adverse reactions can be minimized through multidisciplinary teams, and these teams can respond promptly and accurately if such reactions occur. Furthermore, in-depth research regarding the mechanism of metabolic surgery will facilitate potential noninvasive treatment of obesity and its complications in the future. Overall, we advocate for the establishment of national or regional metabolic surgery registration systems. It is our goal and current intention to explore the long-term effectiveness and best-practice safety of metabolic surgery in practice.
Funding
This study was supported by the Clinical Research Plan of SHDC (SHDC2020CR1017B), Shanghai Municipal Key Clinical Specialty, the National Natural Science Foundation of China (81670791), Municipal Natural Science Foundation of Shanghai (17ZR1421200), Shanghai Key Clinical Center for Metabolic Disease (2017Z01013), and Clinical Retrospective Study of Shanghai Jiao Tong University Aflited Sixth People's Hospital (NHG201912 and YNHG202006). Conflict of interest: none declared.
Conflict of interest: none declared.
References
1. Afshin, A., Forouzanfar, M.H., Reitsma, M.B., et al. (2017). Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. . Med. 377, 13-27.
2. American Diabetes Association. (2018). 7. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes- 2018. Diabetes Care 41(Suppl 1), 565-572.
3. American Diabetes Association. (2019). 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2019. Diabetes Care 42(Suppl 1), S81-S89.
4. American Diabetes Association. (2020). 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1), S89-S97.
5. American Diabetes Association. (2021). 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2021. Diabetes Care 44(Suppl 1), S100- -S110.
6. Chang, D.M., Lee, W.J,. Chen, J.C, et al. (2018). Thirteen-year experience of laparoscopic sleeve gastrectomy: surgica! risk, weight loss, and revision procedures. Obes. Surg. 28, 2991-2997.
7. Chang, H.W., Yang, P.Y, Han, T.l,, et al. (2019). Wernicke encephalopathy concurrent with polyradiculoneuropathy in a young man after bariatric surgery: a case report. Medicine 98, e1 4808.
8. Chang, Y.C., Chao, S.H., Chen, C.C, et al. (2021). The effects of bariatric surgery on renal, neurological, and ophthalmic complications in patients with type 2 diabetes: the Taiwan diabesity study. Obes. Surg. 31, 117-126.
9. Chen, J,, Yu, H., Chen, L., et al. (2017). Effect of Roux-en-Y gastric bypass on carotid intima-media thickness in Chinese obese patients with type 2 diabetes. Surg. Obes. Relat. Dis. 13, 1530-1535.
10. Chen, J.H., Wei, Y.F., Chen, C.Y., et al. (2021). Decreased long-term respira- tory infection risk after bariatric surgery: a comprehensive national cohort study. Obes. Surg. 31, 499- 507.
11. Chen, S.B., Lee, Y.C., Ser, K.H., et al. (2009). Serum C-reactive protein and white blood cell count in morbidly obese surgical patients. Obes. Surg.19, 461-466.
12. Chen, W., Yin, H., Zhang, N., et al. (2020a). Changes of resting energy expenditure in type 2 diabetes rats after Roux-en-Y gastric bypass. Obes. Surg.30, 2994- 3000.
13. Chen, W., Zhang, G.. Dong, Z., et al. (2020b). Cardia gastric cancer in the gastric pouch 5 years after gastric bypass: a case report. Obes. Surg. 30, 2021- -2025.
14. Chen, X., and Kong, X. (2018). Diabetes remission and relapse after metabolic surgery. }. Diabetes Invest. 9, 1237-1238.
15. Dai, X., Zhao, W., Zhan, J., et al. (2017). B cells present skewed profile and lose the function of supporting T cell infammation after Roux.en-Y gastric bypass. Int. Immunopharmacol.43, 16-22.
16. Di, I., Zhang, H., Yu, H., et al. (2016). Effect of Roux-en-Y gastric bypass on the remission of type 2 diabetes: a 3-year study in Chinese patients with a BMI <30 kg/m2. Surg. Obes. Relat. Dis. 12, 1357- 1363.
17. Ding, D., and Zheng, C. (2016). History and development trend of minimally invasive surgical treatment for obesity and diabetes in China. Zhonghua Wei Chang Wai Ke Za Zhi 19, 854-856.
18. Dongiovanni, P.. Paolini, E., Corsini, A., et al. (2021). NAFLD or MAFLD diagnoses and cardiovascular diseases: from epidemiology to drug approaches. Eur. ). Clin. Invest. 51, e13519.
19. Du, X., Fu, X.H., Peng, B.Q., et al. (2018a). Resolution of metabolic syndrome and related metabolic disorders after bariatric surgery: comparison of sleeve gastrectomy and gastric bypass. Surg. Obes. Relat. Dis. 14, 1348- -1356.
20. Du, X., Fu, X.H., Shi, L, et al. (2018b). Effects of laparoscopic Roux-en-Y gastric bypass on Chinese type 2 diabetes mellitus patients with different levels of obesity: outcomes after 3 years' follow-up. Obes. Surg. 28,702-711.
21. Du, X., Zhang, S.Q, Zhou, H.X., et al. (201 6). Laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass for morbid obesity: a 1:1 matched cohort study in a Chinese population. Oncotarget 7, 76308- 76315.
22. Du, X., Zhou, H.X, Zhang, S.Q., et al. (2017). A comparative study of the metabolic effects of LSG and LRYGB in Chinese diabetes patients with BMI <35 kg/m2. Surg. Obes. Relat. Dis. 13, 189-197.
23. Emous, M,Wolffenbuttel, B.H.R, Totte, E., et al. (2017). The short- to mid-term symptom prevalence of dumping syndrome after primary gastric-bypass surgery and its impact on health-related quality of life.
Surg. Obes. Relat. Dis. 13, 1489-1 500.
24. Feng, W., Yin, T., Chu, X., et al. (2019). Metabolic effects and safety of Roux-en-Y gastric bypass surgery vs. conventional medication in obese Chinese patients with type 2 diabetes. Diabetes Metab. Res. Rev. 35, e3138.
25. Gong, K., Li, K., Zhang, N., et al. (2017). Gastric bypass procedure for type 2 diabetes patients with BMI <28 kg/m2. Surg. Endosc. 31, 1172-1179.
26. Gronroos, S., Helmio, M., Juuti, A., et al. (2021). Effect of laparoscopic sleeve gastrectomy Vs Roux-en-Y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg.156, 137-146.
27. Guan, B., Yang, I.. Chen, Y,, et al. (2018). Nutritional deficiencies in Chinese patients undergoing gastric bypass and sleeve gastrectomy: prevalence and predictors. Obes. Surg. 28, 2727-2736.
28. Guo, W., Han, H., Wang, Y.. et al. (2016). miR- 200a regulates Rheb-mediated amelioration of insulin resistance after duodenal-jejunal bypass. Int. J. Obes.40, 1222-1232.
29. Guo, Y., Liu, C.Q., Shan, C.X., et al. (2017). Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: increased diversity and associations of discriminant genera with
metabolic changes. Diabetes Metab. Res. Rev.33, doi: 10.1002/dmrr. 2857.
30. Han, H, Chen, L., Wang, M., et al. (2019a). Benefts of bariatric surgery in patients with acute ischemic stroke-a national population-based study. Surg. Obes. Relat. Dis. 15, 1934-1942.
31. Han, H., Zhu, T., Guo, Y.. et al. (2019b). Impact of prior bariatric surgery on outcomes of hospitalized patients with heart failure: a population-based study. Surg. Obes. Relat. Dis. 15, 469- 477.
32. Hans, P.K, Guan, W., Lin, S., et al. (2018). Long-term outcome of laparoscopic sleeve gastrectomy from a singte center in mainland China. Asian J. Surg. 41, 285-290.
33. Hatoum, IJ.. and Kaplan, L.M. (2013). Advantages of percent weight loss as a method of reporting weight loss after Roux.en-Y gastric bypass. Obesity 21, 1519-1525.
34. He, B.. Chen, L, Yu, C., et al. (2014). Roux-en-Y gastric bypass increases hepatic and peripheral insulin sensitivity in rats with type 2 diabetes mellitus. Surg. Obes. Relat. Dis. 10, 485-493.
35. He, B., Liu, L, Yu, C., et al. (2015). Roux- en-Y gastric bypass reduces lipid overaccumulation in liver by upregulating hepatic autophagy in obese diabetic rats. Obes, Surg. 25, 109-118.
36. He, B.. Piao, D., Yu, C.. et al. (2013). Amelioration in hepatic insulin sensitivity by reduced hepatic lipid accumulation at short-term after Roux-en-Y gastric bypass surgery in type 2 diabetic rats. Obes. Surg. 23,
2033- -2041.
37. Hou, C.C, Shyu, R.S., Lee, W.., et al. (2013). Improved renal function 12 months after bariatric surgery. Surg. Obes. Relat. Dis. 9, 202-206. Hsieh, M.F, Chen, J.H,, Su, Y.C, et al. (2020). The increasing possibility of pregnancy postbariatric surgery: a comprehensive national cohort study in Asian population. Obes. Surg. 31, 1022-1029.
38. Hsu, C.C., Almulaif, A.. Chen, J.C., et al. (2015). Effect of bariatric surgery vs medical treatment on type 2 diabetes in patients with body mass index lower than 35: five-year outcomes. JAMA Surg. 150, 1117-1124.
39. Huang, C.K., Garg, A., Kuao, H.C, et al. (201 5). Bariatric surgery in old age: a comparative study of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in an Asia centre of excellence. }. Biomed. Res. 29, 118-124.
40. Huang, C.K., and Katakwar, A. (2020). Sleeve plus procedures: need of time. Surg. Today 50, 419-422.
41. Hung, S.L, Chen, C.Y., Chin, W.L, et al. (2021). The long-term risk of cardiovascular events in patients following bariatric surgery compared to a non-surgical population with obesity and the general population: a comprehensive national cohort study. Langenbecks Arch. Surg. 406, 189-196.
42. Hylemon, P.B., Zhou, H., Pandak, W.M., et al. (2009). Bile acids as regulatory molecules. J. Lipid Res.50, 1509-1520.
43. Jiang, F., Zhu, H., Zheng, X., et al. (2014). Duodenaljejunal bypass for the treatment of type 2 diabetes in Chinese patients with an average body
mass index <24 kg/m2. Surg. Obes. Relat. Dis. 10, 641-646.
44. Kam, H., Tu, Y., Pan, J., et al. (2020). Comparison of four risk prediction models for diabetes remission after Roux-en-Y gastric bypass surgery in obese Chinese patients with type 2 diabetes mellitus. Obes. Surg. 30,2147-2157.
45. Ke, Z., Li, F., Chen, J., et al. (2017). Effects of laparoscopic Roux-en-Y gastric bypass for type 2 diabetes mellitus: comparison of BMI >30 and < 30 kg/m2. Obes. Surg. 27, 3040-3047.
46. Ke, Z., L, F, Gao, Y., et al. (2021). The use of visceral adiposity index to predict diabetes remission in low BMI Chinese patients after bariatric surgery. Obes. Surg. 31, 805-812. .
47. Kong, X., Tu, Y, Li, B., et al. (2019). Roux-en-Y gastric bypass enhances insulin secretion in type 2 diabetes via FXR-mediated TRPA1 expression. Mol.Metab.29, 1-11.
48. Kun, L, Pin, Z., lianzhong, D., et al. (2015). Significant improvement of erectile function after Roux.en-Y gastric bypass surgery in obese Chinese men with erectile dysfunction. Obes. Surg. 25, 838-844.
49. Lazzati, A., Bechet, S., Jouma, S., et al. (2020). Revision surgery after sleeve gastrectomy: a nationwide study with 10 years of follow-up. Surg. Obes. Relat. Dis. 16, 1497-1 504.
50. Lee, M.H., Almalki, 0.M., Lee, W.J.. et al. (2020). Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: long-term result and recurrence of diabetes. Obes. Surg. 30, 3669- -3674.
51. Lee, M.H., Lee, W.., Chong, K., et al. (2015a). Predictors of iong-term diabetes remission after metabolic surgery. J. Gastrointest. Surg. 19, 1015-1021.
52. Lee, W... Almulaifi, A., Tsou, J. et al. (201 5b). Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg. Obes. Relat. Dis. 11, 991-996.
53. Lee, W... Chong, K., Chen, S.C., et al. (2016). Preoperative prediction of type 2 diabetes remission after gastric bypass surgery: a comparison of DiaRem Scores and ABCD scores. Obes. Surg. 26, 2418- -2424.
54. Lee, W... Chong, K., Ser, K.H., et al. (2011). Gastric bypass vs sleeve gastrec. tomy for type 2 diabetes mellitus: a randomized controlled trial. Arch. Surg.146, 143-148.
55. Lee, W... Chong, K., Ser, K.H., et al. (2012). C-peptide predicts the remission of type 2 diabetes after bariatric surgery. Obes. Surg. 22, 293-298.
56. Lee, W.., Huang, M.T., Wang, W., et al. (2004). Effects of obesity surgery on the metabolic syndrome. Arch. Surg. 139, 1088-1092.
57. Lee, W.J., Pok, E.H, Almulaifi, A., et al. (201 5c). Medium-term results of laparoscopic sleeve gastrectomy: a matched comparison with gastric bypass. Obes. Surg. 25, 1431-1438.
58. Li, J.. Wang, Y, Zhou, Y., et al. (2012). Gastric bypass surgery alters the mechanisms of insulin resistance in the adipose tissue of GK rats. Mol. Med. Rep.6, 1111-1116.
59. Li, M., Cao, D., Liu, Y, et al. (2020a). Alterations in the liver fat fraction features examined by magnetic resonance imaging following bariatric surgery: a sel-controlled observational study. Obes. Surg. 30, 1917-1928.
60. Li, M., Li, H., Zhou, Z.. et al. (2016). Duodenal:jejunal bypass surgery ameliorates glucose homeostasis and reduces endoplasmic reticulum stress in the liver tissue in a diabetic rat model. Obes. Surg. 26, 1002-1009.
61. Li, M., Liu, Y., Zhang, S.. et al. (2021a). Greater China metabolic and bariatric surgery database registry report (2020). Chin. ). Pract. Surg. 41, 533-542.
62. Li, M., Liu, Y, Zeng, N., et al. (2021b). Alterations in the serum urate concentrations after bariatric surgery: a short-term prospective observational study. Obes. Surg. 31, 1688-1695.
63. Li, N, Yan, Q.T., Jing, Q.. et al. (2020b). Duodenal-jejunal bypass ameliorates type 2 diabetes mellitus by activating insulin signaling and improving glucose utilization in the brain. Obes. Surg. 30, 279-289.
64. Li, S., Li, Y., and Tian, H. (2015). Higher baseline BMI is associated with greater reduction of apnea-hypopnea index after bariatric surgery. Obes. Surg. 25, 1491-1493.
65. Li, W., Zhu, L, Mo, Z.. et al. (2014). Effect of laparoscopic Roux.en-Y gastric bypass on body composition and insulin resistance in Chinese patients with type 2 diabetes mellitus. Obes. Surg. 24, 578-583.
66. Li, Y., Zhang, H., Tu, Y, et al. (2019). Monogenic obesity mutations lead to
less weight loss after bariatric surgery: a 6-year follow-up study. Obes.
Surg. 29, 1169-1173.
67.Liang, Z., Wu, Q, Chen, B., et al. (2013). Effect of laparoscopic Roux.en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res. Clin. Pract. 101, 50- 56.
68. Lin, C.H., Liao, W.L, Wu, C.C., et al. (2020). Nutritional status of obese Taiwanese before bariatric-metabolic surgery and their serum 25-hydroxy-vitamin D concentrations for maximal suppression of parathyroid hormone. Obes. Surg. 30, 3940-3946.
69. Liu, F., Di, 1.. Yu, H., et al. (2017a). Effect of Roux.en-Y gastric bypass on thyroid function in euthyroid patients with obesity and type 2 diabetes. Surg.Obes. Relat. Dis. 13, 1701-1707.
70.Liu, F., Tu, Y., Zhang, P.,. et al. (2018a). Decreased visceral fat area correlates with improved total testosterone levels after Roux-en-Y gastric bypass in obese Chinese males with type 2 diabetes: a 12-month follow-up. Surg. Obes. Relat. Dis. 14, 462- -468.
71. Liu, H.. Zhang, H., Wang, X., et al. (2018b). The family Coriobacteriaceae is a potential contributor to the beneficial effects of Roux-en-Y gastric bypass on type 2 diabetes. Surg. Obes. Relat. Dis. 14, 584-593.
72. Liu, H, Zhang, H., Wang, X., et al. (2019a). Alterations of DNA methylation profile in proximal jejunum potentially contribute to the beneficial effects of gastric bypass in a diabetic rat model. Surg. Obes. Relat. Dis. 15,1291-1298.
73. Liu, R., Hong, )., Xu, X., et al. (2017b). Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med.23, 859- -868.
74. Liu, S.. Cao, D.,. Ren, Z., et al. (2020). The relationships between bariatric surgery and sexual function: current evidence based medicine. BMC Urol. 20,150.
75. Liu, S.Y., Wong, S.K., Lam, C.C., et al. (2015). Long-term results on weight loss and diabetes remission after laparoscopic sleeve gastrectomy for a morbidly obese Chinese population. Obes. Surg. 25, 1901-1908.
76. Liu, W., Zhang, H., Han, X., et al, (2019b). Uric acid level changes after bariatric surgery in obese subjects with type 2 diabetes mellitus. Ann. Transl. . Med.7, 332.
77. Lu, C., Li, Y., Li, L, et al. (2020). Alterations of serum uric acid level and gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a hyperuricemic rat model. Obes. Surg. 30, 1799-1807.
78. Lu, C.W., Chang, Y.K., Lee, Y.H., et al. (201 8). Increased risk for major depressive disorder in severely obese patients after bariatric surgery- a 12-year nationwide cohort study. Ann. Med.50, 605-612.
79. Lu, J., Bai, Z., Chen, Y, et al. (2021). Effects of bariatric surgery on serum uricacid in people with obesity with or without hyperuricaemia and gout: a retrospective analysis. Rheumatology 60, 3628-3634.
80. Luo, D., Yang, Q., Zhou, L., et al. (2020). Comparative effects of three kinds of bariatric surgery: a randomized case-control study in obese patients. Diabetes Ther. 11, 175-183.
81. Luo, P., Yu, H., Zhao, X., et al. (2016). Metabolomics study of Roux.en-Y gastric bypass surgery (RYGB) to treat type 2 diabetes patients based on ultraperformance liquid chromatography-mass spectrometry. J. Proteome Res.15, 1288-1299.
82. Maghrabi, A.H., Wolski, K., Abood, B., et al. (2015). Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity 23,2344 -2348.
83. Mangan, A., Le Roux, C.W., Miller, N.G., et al. (2019). lron and vitamin D/calcium deficiency after gastric bypass: mechanisms involved and strategies to improve oral supplement disposition. Curr. Drug Metab. 20,244-252.
84. Mao, R., Guo, P.. Lin, Z., et al. (2021). Nomograms for predicting non-remission in patients who underwent bariatric surgery: a multicenter retrospective study in China. Obes. Surg. 31, 1967-1978.
85. Mazidi, M., Gao, H.K., Hui, H., et al. (2017a). Changes in adiposity and other cardiometabolic risk factors following Roux-en-Y gastric bypass: a 12 -month prospective cohort study in Chinese patients. Indian J.
Gastroenterol. 36, 258- -262.
86. Mazidi, M., Gao, H.K., Li, L, et al. (2017b). Effects of Roux-en.Y gastric bypass on insulin secretion and sensitivity, glucose homeostasis, and diabetic control: a prospective cohort study in Chinese patients. Surgery 161, 1423-1429.
87. Nautiyal, H.K., Guan, W., Lin, S., et al. (2020). Preoperative predictors of early relapse/ no-remission of type-2 diabetes after metabolic surgery in Chinese patients. Clin. Obes. 10, e12350.Nie, X., Chen, J., Ma, X., et al. (2020). A metagenome-wide association study of gut microbiome and visceral fat accumulation. Comput. Struct. Biotechnol. J. 18, 2596-2609.
88. Nora, M., Guimaraes, M., Almeida, R., et al. (2014). Excess body mass index loss predicts metabolic syndrome remission after gastric bypass.Diabetol. Metab. Syndr. 6, 1. Peterli, R., Wolnerhanssen, B.K., Peters, T., et al. (2018). Effect of laparoscopic sleeve gastrectomy Vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. IAMA 319, 255-265.
89. Qian, B., Zhou, X., Li, B., et al. (2014). Reduction of pancreatic B-cell dedifferentiation after gastric bypass surgery in diabetic rats. J. Mol. Cell Biol. 6, 531-534.
90. Rubino, F., Nathan, D.M., Eckel, R.H., et al. (2017). Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Obes. Surg. 27, 2-21. Rubino, F., Shukla, A., Pomp, A., et al. (2014). Bariatric, metabolic, and diabetes surgery: what's in a name? Ann. Surg. 259, 117-122.
91. Ruze, R., Li, J., Xu, Q., et al. (2020). Sleeve gastrectomy ameliorates alveolar structures and surfactant protein expression in lungs of obese and diabetic rats. Int. J. Obes.44, 2394-2404.
92. Schauer, P.R., Bhatt, D.L, Kinwan, J.P.,. et al. (2017). Bariatric surgery versus intensive medical therapy for diabetes- 5-year outcomes. N. Engl. I. Med. 376, 641-651.
93. Shao, Y., Ding, R., Xu, B., et al. (2017). Alterations of gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in Sprague -Dawley rats. Obes. Surg. 27, 295-302.
94. Shao, Y., Shen, Q., Hua, R., et al. (2018). Effects of sleeve gastrectomy on .the composition and diurnal oscillation of gut microbiota related to the metabolic improvements. Surg. Obes. Relat. Dis. 14, 731-739. .
95. Shi, X, Karmali, S., Sharma, A.M., et al. (2010). A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes. Surg. 20, 1171-1177. Staels, B., and Fonseca, V.A. (2009). Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care 32(Suppl 2), S237-S245.
96. Sun, D., Liu, S., Zhang, G., et al. (2014). Sub-sleeve gastrectomy achieves good diabetes control without weight loss in a non-obese diabetic rat model. Surg. Endosc. 28, 1010-1018.
97. Sun, W., Zhang, Y., Shen, Q.. et al. (2019). Prevalence and risk factors for symptoms suggestive of hypoglycemia and early dumping syndrome after sleeve gastrectomy. Surg. Obes. Relat. Dis. 15, 1439-1446.
98. Tai, C.M., Huang, C.K., Hwang, J.C., et al. (2012). Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes. Surg. 22, 1016-1021.
99.Tai, C.M., Lee, Y.C.. Wu, M.S, et al, (2009). The effect of Roux.en-Y gastric bypass on gastroesophageal reflux disease in morbidly obese Chinese patients. Obes. Surg. 19, 565- 570.
100.Tang, Q., Sun, 2., Zhang, N, et al. (2016). Cost- effectiveness of bariatric surgery for type 2 diabetes mellitus: a randomized controlled trial in China. Medicine 95, e3522.
101. Tu, Y., Pan, Y.. Han, )., et al. (2021). A total weight loss of 25% shows better predictivity in evaluating the eficiency of bariatric surgery. Int. I. Obes. 45, 396- -403.
102. Tu, Y., Wang, L., Wei, L, et al. (2019). Cost-utility of laparoscopic Roux-en-Y gastric bypass in Chinese patients with type 2 diabetes and obesity with a BMI≥27.5 kg/m2: a multi-center study with a 4-year follow-up of surgical cohort. Obes. Surg. 29, 3978-3986.
103. Tu, Y., Yu, H., Bao, Y., et al. (2015). Baseline of visceral fat area and decreased body weight correlate with improved pulmonary function after Roux-en-Y gastric bypass in Chinese obese patients with BMI 28-35 kg/m2 and type 2 diabetes: a 6-month follow-up. BMC Endocr. Disord. 15,
104. Van De Laar, A. (2012). Bariatric Outcomes Longitudinal Database (BOLD) suggests excess weight loss and excess BMI loss to be inappropriate outcome measures, demonstrating better alternatives. Obes. Surg. 22, 1843- 1847.
105. Wan, B., Fang, N., Guan, W., et al. (2019). Cost effectiveness of bariatric surgery versus medication therapy for obese patients with type 2 diabetes in China: a Markov analysis. J. Diabetes Res.2019, 1341963.
106. Wang, C., He, B., Piao, D., et al. (2016a). Roux-en.Y esophagojejunostomy ameliorates renal function through reduction of renal infammatory and fi. brotic markers in diabetic nephropathy. Obes. Surg. 26, 1402-1413.
107. Wang, C., Pang, S., jiang, Q., et al. (2013). Treatment with acarbose in severe hypoglycaemia due to late dumping syndrome. West Indian Med. J 62, 861-863.
108. Wang, C., Ren, Y, Chen, 1., et al. (2008). Fatal fulminant pancreatitis after laparoscopic gastric bypass surgery. Obes. Surg. 18, 1498- 1501.
109. Wang, C., Zhang, H., Yu, H., et al. (2020a). Roux-en-Y gastric bypass for T2D treatment in Chinese patients with low BMl: 5-year outcomes. Obes. Surg. 30, 2588-2597.
110. Wang, G., Wang, Q.. Bai, 1.. et al. (2020b). RYGB increases postprandial gastric nesfatin-1 and rapid relieves NAFLD via gastric nerve detachment. PLoS One 15, e0243640.
111. Wang, G., Wang, Q., Bai, J.. et al. (2020c). Upregulation of intestinal NLRP6 inflammasomes after Roux-en-Y gastric bypass promotes gut immune homeostasis. Obes. Surg. 30, 327-335.
112. Wang, G., Zhu, L, Li, W., et al. (2016b). Can low BM| Chinese patients with type 2 diabetes beneft from laparoscopic Roux-en-Y gastric bypass surgery? Surg. Obes. Relat. Dis. 12, 1890-1895.
113. Wang, L.. Sang, Q., Zheng, X., et al. (2021). Early weight loss following laparoscopic sleeve gastrectomy is predictive of long-term weight loss in morbidly obese Chinese. Obes. Surg. 31, 820-828.
114. Wang, L, Wang, J, and Jiang, T. (2019a). Effect of laparoscopic sleeve gastrectomy on type 2 diabetes mellitus in patients with body mass index less than 30 kg/m2. Obes. Surg. 29, 835-842.
115.Wang, W., Mcgreevey, W.P., Fu, C., et al. (2009). Type 2 diabetes mellitus in China: a preventable economic burden. Am. J. Manag. Care 15, 593-601.
116.Wang, X., Chang, X.S., Gao, L, et al. (2016c). Effectiveness of laparoscopic sleeve gastrectomy for weight loss and obesity-associated co-morbidities: a 3-year outcome from Mainland Chinese patients. Surg. Obes. Relat. Dis. 12, 1305-1311.
117. Wang, X., Su, C., Shen, X., et al. (2019b). Risk factors for relapse of hyperglycemia after laparoscopic Roux-en-Y gastric bypass in T2DM obese patients: a 5-year follow-up of 24 cases. Obes. Surg. 29, 1164-1168.
118. Wang, Y.. Chen, J.. and Wu, X.T. (2020d). No effect on change in fasting ghrelin at≤12 months and increased at≥24 months after Roux-en-Y gastric bypass. Obes. Surg. 30, 342-345.
119. Wei, J.H, Chou, R.H., Huang, P.H., et al. (201 8a). Metabolic surgery ameliorates cardiovascular risk in obese diabetic patients: influence of different surgical procedures. Surg. Obes. Relat. Dis. 14, 1832-1840.
120. Wei, J.H., Lee, W... Chong, K., et al. (201 8b). High incidence of secondary hyperparathyroidism in bariatric patients: comparing different procedures. Obes. Surg. 28, 798-804.
121. Wei, Y, Wu, T., Tong, D.K.H., et al. (2020). Improvement in patient-reported outcomes in Chinese adults after bariatric surgery: 1-year follow-up of a
prospective cohort. Surg. Obes. Relat. Dis. 16, 1563-1572.
122. Wen, Y., Lin, N., Yan, H.T., et al. (2015). Down-regulation of renal gluconeogenesis in type II diabetic rats following Roux en-Y gastric bypass surgery: a potential mechanism in hypoglycemic effect. Obes. Facts 8, 110-124.
123. Wong, C.K.H., Wu, T., Wong, S.K.H, et al. (2021). Effects of bariatric surgery on kidney diseases, cardiovascular diseases, mortality and severe hypo-
glycaemia among patients with Type 2 diabetes mellitus. Nephrol. Dial. Transplant.36, 1440-1451.
124. Wu, D., Wang, L, and Jiang, T. (2020a). Efficacy comparison of laparoscopic sleeve gastrectomy in type 2 diabetes patients with a BMI 30- 34.9 kg/m2 versus BMI <30 kg/m2. Surg. Endosc.35, 1544-1550.
125. Wu, E., Luk, A., Wong, S.K., et al. (2016). Health-related quality of life after bariatric surgery and its correlation with glycaemic status in Hong Kong Chinese adults. Obes. Surg. 26, 538- 545.
126. Wu, T., Wong, S.K.H, Law, B.T.T., et al. (2020b). Five-year effectiveness ol bariatric surgery on disease remission, weight loss, and changes of metabolic parameters in obese patients with type 2 diabetes: a
population-based propensity score-matched cohort study. Diabetes Metab. Res. Rev.36, e3236.
127. Wu, T., Wong, S.K.H., Law, B.T.I., et al. (2021). Bariatric surgery is expensive but improves co-morbidity: 5-year assessment of patients with obesity and type 2 diabetes. Br. J. Surg.108, 554-565.
128. Xia, J., He, Q.. He, M., et al. (201 9). Residual gastric dilatation interferes with metabolic improvements following sleeve gastrectomy by upregulating the expression of sodium-glucose cotransporter-1. Obes. Surg. 29, 3324- -3333.
129. Xu, H., Zhang, P.. Han, X., et al. (2016). Sex effect on obesity indices and metabolic outcomes in patients with obese obstructive sleep apnea and type 2 diabetes after laparoscopic Roux-en-Y gastric bypass surgery: a preliminary study. Obes. Surg. 26, 2629- -2639.
130. Xu, L, Yin, )., Mikami, D.J.. et al. (2015). Effectiveness of laparoscopic Roux-en-Y gastric bypass on obese classI type 2 diabetes mellitus patients. Surg. Obes. Relat. Dis. 11, 1220-1226.
131. Yan, H., Tang, L, Chen, T., et al. (2013a). Defining and predicting complete remission of type 2 diabetes: a short-term efcacy study of open gastric bypass. Obes. Facts 6, 176-184.
132. Yan, S., Sun, F., Li, Z., et al. (2013b). Reduction of intestinal electrogenic glucose absorption after duodenojejunal bypass in a mouse model. Obes. Surg.23, 1361-1369.
133. Yan, Y., Zhou, Z., Kong, F. et al. (2016). Roux.en-Y gastric bypass surgery suppresses hepatic gluconeogenesis and increases intestinal gluconeo- genesis in a T2DM rat model. Obes. Surg. 26, 2683-2690.
134. Yang, J.. Feng, X., Zhong, S, et al. (2014). Gastric bypass surgery may im. prove beta cell apoptosis with ghrelin overexpression in patients with BMI≥32.5 kg/m2. Obes. Surg. 24, 561-571.
135. Yang, J., Gao, Z., Williams, D.B., et al. (2018). Effect of laparoscopic Roux.en-Y gastric bypass versus laparoscopic sleeve gastrectomy on fasting gastrointestinal and pancreatic peptide hormones: a prospective nonrandomized trial. Surg. Obes. Relat. Dis. 14, 1521-1529.
136. Yang, J.. Gao, Z., Yang, W., et al. (2017). Effect of sleeve gastrectomy on thyroid function in Chinese euthyroid obese patients. Surg. Laparosc. Endosc. Percutan. Tech. 27, e66-e68.
137. Yang, J.. Wang, C., Cao, G., et al. (2015). Long-term effects of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m2. BMC Surg. 15, 88.
138. Yang, K., Zhang, X., Zhou, Y., et al. (2020). Changes in serum nesfatin-1 after laparoscopic sleeve gastrectomy are associated with improvements in nonalcoholic fatty liver disease. Diabetes Metab. Syndr, Obes. 13, 1459-1464.
139. Yang, K., Zhou, Y.. Wang, M., et al. (2019). Status of the field of bariatric surgery: a National Survey of China in 2018. Obes. Surg. 29, 1911-1921.
140. Yeh, P.S., Lee, Y.C, Lee, W.J, et al. (2010). Clinical predictors of obstructive sleep apnea in Asian bariatric patients. Obes. Surg. 20, 30-35.
141. Yin, J., Xu, L., Mao, Z., et al. (2014). Laparoscopic Roux-en-Y gastric bypass for type 2 diabetes mellitus in nonobese Chinese patients. Surg. Laparosc. Endosc. Percutan. Tech. 24, e200-e206.
142. Yin, X., Qian, J.. Wang, Y., et al. (2019). Short-term outcome and early effect on blood pressure of laparoscopic sleeve gastrectomy in morbidly obese patients. Clin. Exp. Hypertens. 41, 622-626.
143. Yong, W., Shibo, W., and lingang, L. (2012). Remission of insulin resistance in type 2 diabetic patients after gastric bypass surgery or exenatide therapy. Obes. Surg. 22, 1060-1067.
144. Yu, H., Chen, J., Lu, J., et al. (2016a). Decreased visceral fat area correlates with improved arterial stiffness after Roux-en-Y gastric bypass in Chinese obese patients with type 2 diabetes mellitus: a 12-month follow-up. Surg. Obes. Relat. Dis. 12, 550-555.
145. Yu, H., Di, J.,. Bao, Y., et al. (2015a). Visceral fat area as a new predictor of short-term diabetes remission after Roux-en-Y gastric bypass surgery in Chinese patients with a body mass index less than 35 kg/m2. Surg. Obes. Relat. Dis. 11, 6-11.
146. Yu, H., Du, R.. Zhang, N., et al. (2016b). lron-deficiency anemia after laparoscopic Roux-en-Y gastric bypass in Chinese obese patients with type 2 diabetes: a 2-year follow-up study. Obes. Surg. 26, 2705-2711.
147. Yu, H., Li, Q, Zhang, M., et al. (2019). Decreased leptin is associated with alterations in thyroid-stimulating hormone levels after Roux-en-Y gastric bypass surgery in obese euthyroid patients with type 2 diabetes. Obes. Facts 12, 272-280.
148. Yu, H., Ni, Y., Bao, Y., et al. (2015b). Chenodeoxycholic acid as a potential prognostic marker for Roux.en-Y gastric bypass in Chinese obese patients. }. Clin. Endocrinol. Metab. 100, 4222-4230.
149. Yu, H., Zhang, L, Bao, Y., et al. (2016C). Metabolic syndrome after Roux.en-Y gastric bypass surgery in Chinese obese patients with type 2 diabetes. Obes. Surg. 26, 2190- -2197.
150. Yu, H., Zhou, J.. Bao, Y., et al. (2016d). Dual-remission' after Roux-en-Y gastric bypass surgery: glycemic variability cannot always be improved in Chinese obese patients with type 2 diabetes. Surg. Obes. Relat. Dis.12, 1312-1319.
151. Yuan, X., Chen, J., Cheng, Q., et al. (2018). Hepatic expression of Yin Yang 1 (YY1) is associated with the non-alcoholic fatty liver disease (NAFLD) progression in patients undergoing bariatric surgery. BMC Gastroenterol. 18,147.
152. Zhan, J., Huang, L, Ma, H., et al. (2017). Reduced inflammatory responses of fllicular helper T cell promote the development of regulatory B cells after Roux.en-Y gastric bypass. Clin. Exp. Pharmacol. Physiol. 44, 556- 565.
153. Zhang, C., Cai, W., Zhao, H, et al. (2020). Effect of gastric bypass on BMI and tipid metabolism in type 2 diabetes mellitus. Artif. Cells Nanomed. Biotechnol. 48, 903-911.
154. Zhang, H., Di, J., Yu, H, et al. (201 5). The short-term remission of diabetic nephropathy after Roux.en-Y gastric bypass in Chinese patients of T2DM with obesity. Obes. Surg. 25, 1263-1270.
155. Zhang, H., Han, X., Yu, H., et al. (2017a). Effect of Roux.en-Y gastric bypass on remission of T2D: medium-term follow-up in Chinese patients with different BMI obesity class. Obes. Surg. 27, 134-142.
156. Zhang, H., Liu, W., Han, X., et al. (2017b). Effect of laparoscopic Roux-en-Y gastric bypass surgery on thyroid hormone levels in Chinese patients, could it be a risk for thyroid nodules? Obes. Surg. 27, 2619-2627.
157. Zhang, Q., Chen, Y., Li, J.. et al. (2018). A meta-analysis of the effects of bariatric surgery on fracture risk. Obes. Rev.19, 728-736.
158. Zhang, Y., Zhao, H, Cao, Z., et al. (2014). A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes. Surg. 24, 1617-1624.
159. Zhang, X., Wang, Y., Zhong, M., et al. (2016). Duodenaljejunal bypass preferentially elevates serum taurine-conjugated bile acids and alters gut microbiota in a diabetic rat model. Obes. Surg. 6, 1890-1899.
160. Zhao, L., Li, W., Su, Z., et al. (2018). Preoperative fasting C-peptide predicts type 2 diabetes mellitus remission in low-BMI Chinese patients after Roux-en-Y gastric bypass. J. Gastrointest. Surg. 22, 1672-1678.
161. Zhao, L, Ni, Y., Yu, H., et al. (2017a). Serum stearic acid/ palmitic acid ratio as a potential predictor of diabetes remission after Roux.en-Y gastric by- pass in obesity. FASEB J. 31, 1449-1460.
162. Zhao, L, Zhu, L, Su, Z., et al. (2017b). Using the hyperinsulinemic euglycemic clamp to assess insulin sensitivity at 3 months following Roux.en-Y gastric bypass surgery in type 2 diabetes patients with BMI <35 kg/m2 in China. Int. J. Surg. 38, 90-94.
163. Zhao, X., Duan, W., Sun, C., et al. (2017c). Decreased cardiovascular risk after Roux-en-Y gastric bypass surgery in Chinese diabetic patients with obesity. l. Diabetes Res.2017, 5612049.
164. Zhong, M.W., Liu, S.Z., Zhang, G.Y., et al. (2016). Alterations in gut microbiota during remission and recurrence of diabetes after duodenal-jejunal bypass in rats. World J. Gastroenterol. 22, 6706-6715.
165. Zhu, C., Gao, J., Mei, F., et al. (2019a). Reduction in thyroid-stimulating hormone correlated with improved inflammation markers in Chinese patients with morbid obesity undergoing laparoscopic sleeve gastrectomy. Obes. Surg.29, 3954-3965.
166. Zhu, C.. Mei, F., Gao, J.. et al. (201 9b). Changes in inflammatory markers correlated with increased testosterone after laparoscopic sleeve gastrectomy in obese Chinese men with acanthosis nigricans. J. Dermatol. 46,338-342.
167. Zhu, C., Zhang, Y., Zhang, L,, et al. (2019c). Changes in sex hormones after laparoscopic sleeve gastrectomy in Chinese obese men: a 12-month fol- low-up. Obes. Surg. 29, 869-877.
168. Zhu, Z., Yin, J.,. Li, D.C., et al. (2017). Role of microRNAs in the treatment of type 2 diabetes mellitus with Roux.en-Y gastric bypass. Braz. }. Med. Biol. Res.50, e5817.
169. Zou, J., Zhang, P., Yu H., et al. (201 5). Effect of laparoscopic Roux-en-Y gastric bypass surgery on obstructive sleep apnea in a Chinese population with obesity and T2DM. Obes. Surg. 25, 1446-1453.
This article is excerpted from the Metabolic surgery in China: present and future by Wound World.


