1 Introduction
Cognitive impairment and dementia are increasing worldwide and are becoming one of the major public health problems. Projection suggests that the number of people afected by dementia will double every 20 years, reaching 115 million by 2050 [1]. Dementia is one of the leading causes of disability in older people. According to a 2015 study, the average cost of dementia care (over a 5-year period) was $287,038, which is higher than the cost of caring for cancer or even hart diseases [2]. It is estimated that 1/3 of people with cognitive impairment will develop dementia during their lifespan. [3] Early identifcation of patients at increased risk of cognitive impairment can imply preventive therapies at an early stage and protect patients from the development of dementia. Te relationship between cerebrovascular disease and neuropsychological decline has been extensively studied. However, the link between mild cognitive impairment (MCI) and lower extremity artery disease is less elucidated. Te purpose of this study was to determine whether lower extremity artery disease is related to cognitive impairment.
2 Aim of the Study
The aim of this study was to investigate the influence of lower extremity artery disease on cognitive impairment.
3 Materials and Methods
We launch a prospective study to investigate the influence of LEAD on cognition. The study was approved by the Bioethics Committee of the Medical University of Wroclaw (KB 526/2017) and all patients gave their informed consent. Te study was conducted in accordance with the Helsinki Declaration. This study was a part of the prospective research investigating cognitive performance in patient with atherosclerosis in various vascular areas. Twenty patients with LEAD qualified for revascularization procedures were included in the current study. Patients were recruited from vascular surgery department between year 2017 and 2019.
Patients with LEAD classified according to Fountain’s stage IIB have been enrolled into the study. Patients with (1) previous TIA, stroke, amaurosis fugax, (2) depression, (3) previous brain injury or brain surgery, (4) modified Rankin scale > =2, (5) other illnesses affecting the central nervous system have not been included. Te control group consisted of 15 patients without peripheral atherosclerosis, qualified for hernia surgery.
On admission, all patients have been assessed on the basis of their medical history and physical examination, including neurological assessment. The information about comorbidities, risk factor and additional medications were collected. In addition, all patients undergo an ultrasound examination of the carotid arteries according to a standard protocol, by a certified ultrasound specialist, before the procedure and 6 months after the procedure. Each patient in the study group was qualified for revascularization surgery after computer tomography imagining or Duplex Doppler and on the basis of symptoms experienced, such as intermittent claudication of less than 200 m. Only patient qualified for revascularization procedure below inguinal ligament and under local anesthesia were included in the study.
According to the cognitive study protocol, all patients were followed up for 6 months, and after this period, the neurocognitive performance was reassessed.
- 1 Neuropsychological Battery
Before the procedure, all participants underwent a neuropsychological examination. The assessment has been done by the trained specialist in a quiet environment. MoCA and CANTAB tests were used as cognitive tools.
MoCA is a global cognition screening test that tests 7 cognitive domains: visuospatial/executive, naming, delayed memory, attention, language, abstraction and orientation. A maximum number of points is 30. To diagnose MCI, a patient must score less than 26 points. It takes about 10 min.
CANTAB (Cambridge Neuropsychological Test Automated Battery) is a true test battery that measures a wide range of cognitive function. For a more precise neurocognitive assessment, 4 tests had been selected for the presented study:
1) MCT—motor screening task—allows patient to get acquainted with IPAD.
In this test, pink and green crosses are displayed one by one on the screen. A patient’s task is to touch them as quickly and accurately as possible. The results of this task have not been included in the analysis.
2) RT—reaction time—assesses the processing speed and visuospatial functions.
In this test, the patient is instructed to hold the button at the bottom of the screen until the yellow dot appears at the top of the screen. A patient must release the button and touch the yellow dot as quickly and accurately as possible. The outcome measure includes:
1) RITIFMRT: RTI mean fve-choice reactiontime. It represents the average time it took for a subject to release the response button after the presentation of a target stimulus. Measured in milliseconds.
3) SWM—spatial working memory—evaluates working memory and executive function.
A series of boxes with tokens hidden inside them appear on the screen. The patient’s task is to use the elimination process to find these tokens and fill in the empty columns on the right side of the screen. Te task becomes more and more difficult as more and more boxes appear on the screen. It is important that the tokens are never placed twice in the same box so that the patient cannot return to the box where the token has already been found. Outcomes measures include:
1) SWM 468 total errors, which represents selecting windows that have already been found empty and revisiting fields that have already been found as containing a token.
2) SWMSX strategy score, which represents the effectiveness of the actions taken.
4) PAL—Paired Associate Learning-assesses visual memory and new learning.
In this test, the boxes are presented on the screen and opened in random order. Some of them have a special symbol inside. Te patient task is to remember what symbol hides in which box. Te symbol is then displayed at the center of the screen and the patient has to select the box where the symbol was originally located. For the final analysis, the following measures are presented:
1) PALFAM 28: PAL First Attempt Memory Score represents the number of times a subject has
marked the correct box on the first attempt when recalling the pattern locations.
2) PALM PR28: PAL Number of Patterns Reached, which is the number of patterns presented to the subject on the last problem they reached.
3) PALTEA28: PAL Total Errors (Adjusted), which is the number of times the subject chose the incorrect box for a stimulus on assessment problems (PALTE), plus an adjustment for the estimated number of errors they would have made on any problems, attempts and recalls they did not reach.
- 2 Statistical Analysis
All statistical analyses were conducted using the R language, v. 3.6.1. A p value<0.05 was considered statistically significant. Chi-square tests were used for comparing categorical variables. Student’s T-tests were used for comparing non-paired samples. To asses which factors influence cognition, linear regression models were built.
4 Results
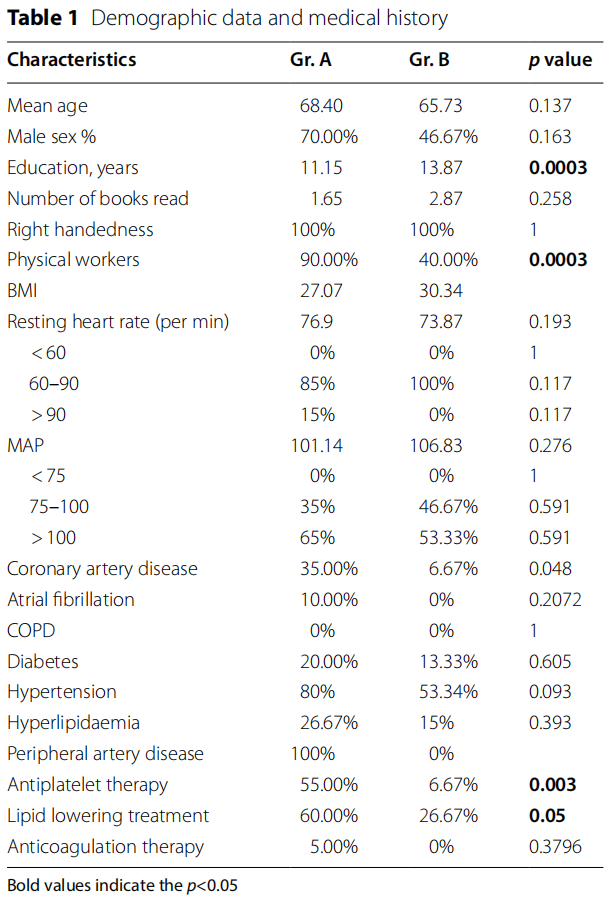
There were 20 patients in the study group with LEAD (group A) and 15 controls (group B). Te patient’s characteristics are summarized in Table 1. Te average age and sex distribution in the groups were similar. Patients with LEAD had lower level of education (p= 0.0003) and were more likely to be physical workers (p= 0.0003). Coronary artery disease was more frequent in patients with LEAD (p=0.048) and that procured differences in terms of medications. Patient with LEAD were more likely to receive antiplatelet therapy (p=0.003) and lipid lowering therapy (p=0.05). There were no differences in mean artery pressure and heart rate between the groups.
- 1 Cognitive Function Results On the First Visit
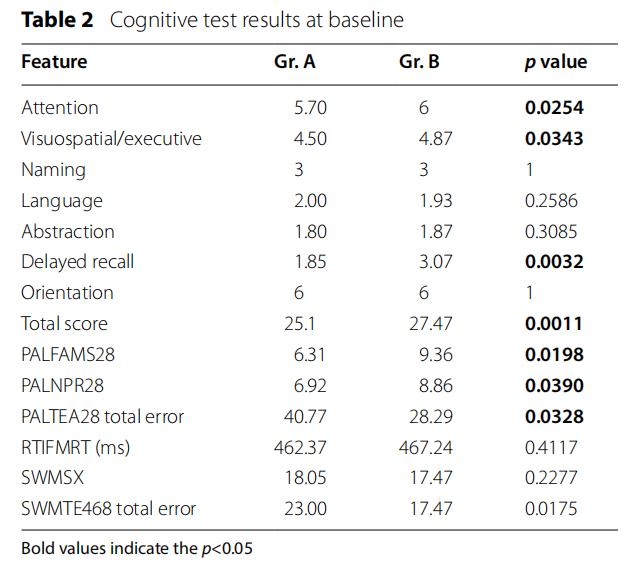
Patients with peripheral artery disease scored fewer points in MoCA test then patients in control group (p =0.0011). Total score was below 26 points what indicates MCI. The significant differences between the study groups were reviled in attention (p=0.0254), visuospatial/executive (p=0.0343) and delayed recall ( p=0.0032). In CANTAB test, patients with peripheral artery disease performed worse in all measurements of Pair Associate Learning (PALFAMS28 p=0.0198; PALNPR28 p =0.0390; PALTEA28 p=0.0328). Te results are listed in Table 2.
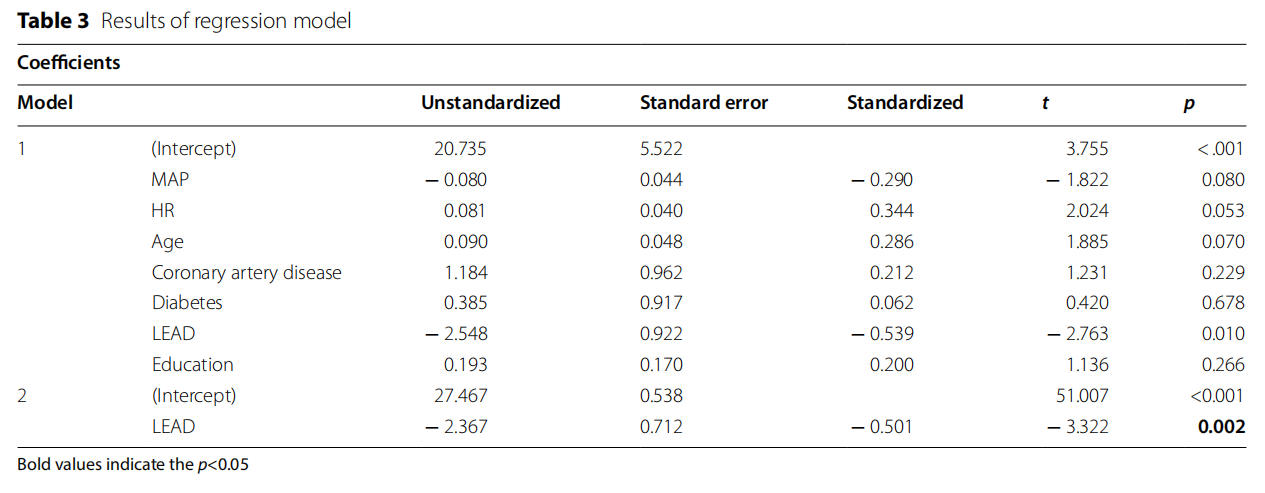
To assess the connection between LEAD and cognitive impairment a linear regression model was build (Table 3). The model showed that lower extremity artery disease was associated with cognitive impairment, independent of potential cofounding factors.
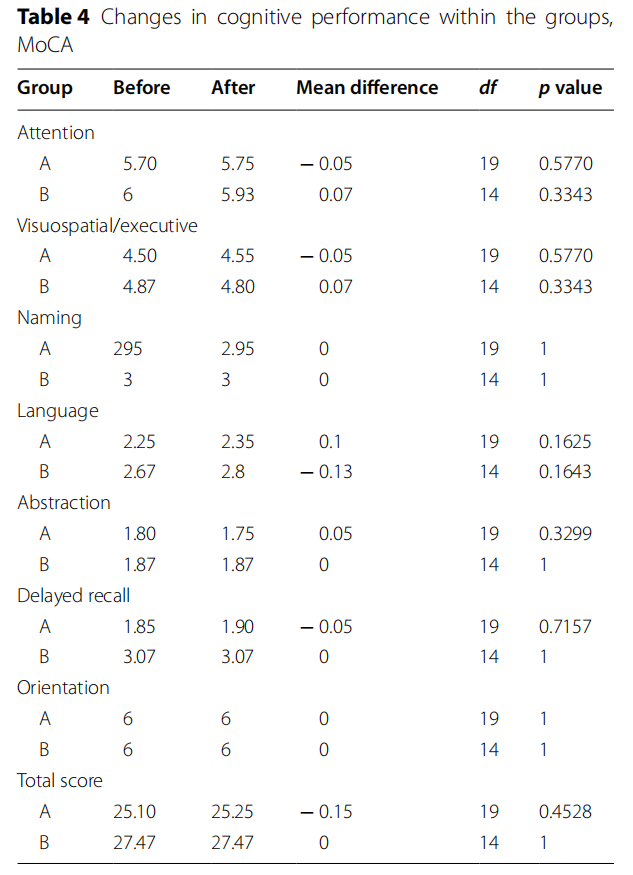
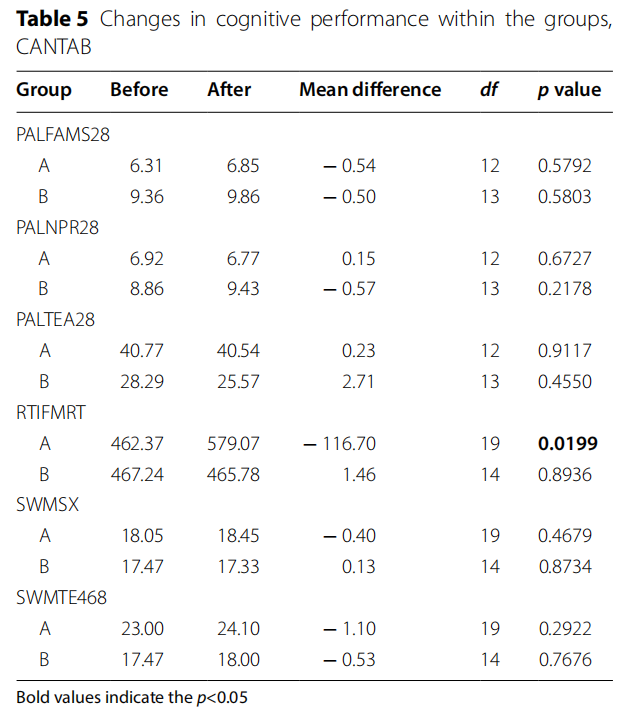
After 6 months on the follow-up visit, there were no major changes in cognition in both groups (Tables 4 and 5). Reaction time was the only parameter that has risen significantly in LEAD group ( p=0.0199).
5 Discussion
Our main finding is that LEAD is associated with cognitive decline regardless of age, gender and common vascular risk factors. The mean MoCA score in the LEAD group was below 26 points what indicates cognitive impairment. The main differences between the study and the control group were noticed in attention, visuospatial/ executive, delayed recall, visual memory and learning. These cognitive domains are often affected in cognitive impairment of vascular origin.
As a marker of generalized atherosclerosis, LEAD leads not only to intermittent claudication and symptoms related to lower extremities but also increase the risk of cardiovascular events including cardiovascular deaths [4–6]. In addition, previously published studies also suggest that patients with LEAD perform worse in neuropsychological evaluation then the healthy patients [7–11].
One large population study in China, the “APAC study” [12], found that a low ABI (below 0.9) is directly related to poorer neuropsychological test scores. 3048 people with no history of stroke were selected in this study. MMSE was performed in each patient and the ankle-brachial index was measured. Reduced ABI was found in 161 people (5.28%), and cognitive impairment in 154 (5.05%).
After performing regression models, it was proved that people with a lowered ABI have a higher risk of cognitive disorders. This correlation was independent of age, gender, education level, or other risk factors for vascular disease. This study showed a correlation between LEAD and cognitive impairment in the Asian population. But their findings do not necessarily apply to white populations, and more research is needed.
Not only the low level of ABI correlates with cognitive functions. In a cross-sectional study [13], it was found that endothelial abnormalities, increased vascular stiffness and microcirculation disorders negatively affects cognitive functions in older population measured by the CANTAB test.
There is a growing body of published research documenting the relationship between cognitive impairment and LEAD. However, the nature of this association remains unclear. One possible explanation is that LEAD is a marker of general atherosclerosis, including cerebral artery disease. Asymptomatic carotid and cerebral atherosclerosis may cause silent infarcts and contribute to cognitive impairment in this mechanism. In our study, only people without carotid artery stenosis were enrolled in the study, and adjustment for cardiovascular disease and vascular risk factors did not change the significance of this correlation. Another possible explanation is that a patient with LEAD may experience subcortical white matter lesions that are also silent. In the Rotterdam Study [14], the mean ABI was significantly lower in people with white matter lesions than in those without. White matter lesions are associated with cognitive decline, and cognitive impairment can be explained by this mechanism [15–17].
Nevertheless, the link between white matter lesions and ABI is not that well established. In another study [ 18] with the use of MRI, the researchers found that the low level of ABI was associated with poorer cognitive functions, irrespective of concomitant cardiovascular risk factors. However, there was no such correlation between low ABI and changes in white matter, volume of brain tissue, or ischemic changes. This evidence shows that there might exist other than general atherosclerosis pathways leading to cognitive impairment in patients with peripheral artery disease and further research is needed.
In our follow-up study, no cognitive changes were found despite adequate restoration of blood flow in the lower extremities and an increase in ABI. This indicates that the poor results in neuropsychological assessment were not correlated with suffering from the LEAD symptom, but the cause is more complex.
Previously published studies used MMSE as a cognitive tool. It is worth notice that MMSE has several limitations because visuospatial/executive functions are not thoroughly tested. These functions are frequently impaired in cognitive impairment of vascular origin [19]. In our study, we use MoCA test. A systematic review showed that the MoCA test outperformed the MMSE test in distinguishing patients with and without cognitive impairment [20].
Our data suggest that LEAD is strongly associated with cognitive impairment. This opens up the possibilities of taking actions to prevent or delayed this disease. In patients with LEAD, the use of secondary prevention, which is appropriate drug therapy and rehabilitation, will not only reduce the number of adverse events of the lower extremities, but may also lead to the maintenance of normal cognitive functions [21, 22].
The sample of this study was relatively low which was a major limitation to the study. Moreover, we did not perform any type the neuroimaging so it was impossible to examine the pathological background. However, the LEAD was diagnosed not only by indirect way with ABI but each patient had arterial imagining.
6 Conclusion
In conclusion, these data suggest that LEAD is associated with cognitive impairment regardless of coexisting vascular risk factors.
Author Contributions
AT—conceptualization, project administration, and writing—original draft. AC—methodology and supervision. MM—validation. TD—data curation and formal analysis. BC—data curation and formal analysis. DJ—supervision and writing—review and editing.
Funding
The study was supported by Wrocław Medical University grant number ESM. E021.17.017.
Data Availability
The data that support the fndings of this study are available from the cor‑ responding author, [AT], upon reasonable request.
Declarations
Conflict of Interest
The author declares that there is no conflict of interest.
Ethical Approval
The study was approved by the Bioethics Committee at the Medical University of Wroclaw (KB 526/2017) and all patients provided informed consent.
Author details
1 Department of Vascular Surgery, 4th Military Teaching Hospital, 5 Weilga street, 50‑981 Wrocław, Poland. 2 Department of Vascular, General and Trans‑ plant Surgery, Faculty of Medicine, Wrocław Medical University, 213 Borowska street, 50‑556 Wrocław, Poland. 3 Institute of Psychology, University of Wroclaw, 1 Dawida street, 50‑529 Wrocław, Poland.
Received: 3 August 2021 Accepted: 7 December 2021
Published online: 10 January 2022
References
1. World Health Organization (2012) Dementia: a public health priority. World Health Organization. https://apps.who.int/iris/bitstream/handle/ 10665/75263/9789241564458_eng.pdf?sequence =1&isAllowed=y.
2. Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163(10):729–36. https://doi.org/10.7326/M15-0381.
3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. https://doi.org/10.1016/j.jalz. 2011.03.008.
4. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26. https://doi.org/10.1161/CIRCRESAHA.116.303849.
5. Ankle Brachial Index Collaboration, Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. https://doi.org/10.1001/jama. 300.2.197.
6. Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufciency of the lower extremities: a critical review. Circulation. 1996;94(11):3026–49. https://doi.org/10.1161/01.cir.94.11.3026.
7. Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: evidence from the Whitehall II Study. J Am Geriatr Soc. 2003;51(10):1445–50. https://doi.org/10.1046/j.1532-5415.2003.51464.x.
8. Tilvis RS, Kähönen-Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strand‑ berg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–74. https://doi.org/10.1093/gerona/59.3.m268.
9. Price JF, McDowell S, Whiteman MC, Deary IJ, Stewart MC, Fowkes FG. Ankle brachial index as a predictor of cognitive impairment in the gen‑ eral population: ten-year follow-up of the Edinburgh Artery Study. J Am Geriatr Soc. 2006;54(5):763–9. https://doi.org/10.1111/j.1532-5415.2006. 00702.x.
10. Woo J, Lynn H, Wong SY, et al. Correlates for a low ankle-brachial index in elderly Chinese. Atherosclerosis. 2006;186(2):360–6. https://doi.org/10. 1016/j.atherosclerosis.2005.07.022.
11. Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Fowkes FG. Cardiovascular diseases and decline in cognitive function in an elderly community population: the Edinburgh Artery Study. Psychosom Med. 2007;69(5):425–34. https://doi.org/10.1097/psy.0b013e318068fce4.
12. Wang A, Jiang R, Su Z, et al. A low ankle-brachial index is associated with cognitive impairment: the APAC study. Atherosclerosis. 2016;255:90–5. https://doi.org/10.1016/j.atherosclerosis.2016.11.005.
13. Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69(9):878–85. https://doi.org/10.1212/01.wnl.0000277657.95487.1c.
14. Bots ML, van Swieten JC, Breteler MM, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet. 1993;341(8855):1232–7. https://doi.org/10.1016/0140-6736(93)91144-b
15. Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77(2):159–65. https://doi.org/10.1136/jnnp. 2004.045567.
16. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157–65. https://doi.org/10.1038/nrneurol.2015.10
17. Alber J, Alladi S, Bae HJ, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement (N Y). 2019;9(5):107–17. https://doi.org/10.1016/j.trci.2019.02.001.
18. Hilal S, Saini M, Tan CS, et al. Intracranial stenosis, cerebrovascular diseases, and cognitive impairment in Chinese. Alzheimer Dis Assoc Disord. 2015;29(1):12–7. https://doi.org/10.1097/wad.0000000000000045.
19. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. https://doi.org/10.1111/j.1532-5415.
20. Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr. 2019;31(4):491– 504 https://doi.org/10.1017/S1041610218001370. 2005.53221.x.
21. Rao R, Jackson S, Howard R. Neuropsychological impairment in stroke, carotid stenosis, and peripheral vascular disease: a comparison with healthy community residents. Stroke. 1999;30(10):2167–73. https://doi. org/10.1161/01.str.30.10.2167.
22. O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. https://doi.org/10.1016/s1474-4422(03) 00305-3 (PMID: 12849265).
*Correspondence: 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。
1 Department of Vascular Surgery, 4th Military Teaching Hospital, 5 Weilga street, 50‑981 Wrocław, Poland
Full list of author information is available at the end of the article
© The Author(s) 2022. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
This article is excerpted from the Artery Research (2022) 28:9–14 by Wound World.


