Wound chronicity was probably first mentioned in literature in 1953 in reference to wounds that were difficult to heal or did not follow a normal healing process (Greeley, 1953). Terminology used includes hard-to-heal wounds, difficult to heal wounds, non-healing wounds and complex wounds (Kyaw et al, 2017). However, most papers do not provide a clear cut-off duration to define the chronicity of wounds (Kyaw et al, 2017).
Chronic wounds, including, lower-limb ulcers, diabetic foot ulcers (DFUs) and pressure injuries (PIs), present features of chronicity from the onset, while other wounds may initially start as acute, like burns, traumatic or postoperative wounds, and become chronic or hard to heal after weeks of stagnation, either due to the patient’s general condition or inappropriate global management or local care (Atkin et al, 2019). These wounds “start by either direct trauma to tissue already compromised by underlying pathology or by breakdown of tissue under unbroken skin” and are “characterised by a physiological barrier to recovery before the breach in the skin appears, an underlying pathophysiology, chronic inflammation and a mostly unpredictable healing trajectory” (Atkin et al, 2019).
It is estimated that the prevalence of chronic wounds is between 1% and 2% in developed countries with the most prevalent wounds being venous leg ulcers (VLUs), pressure injuries and DFUs in people aged over 60, where some of the wounds may not heal completely for a year or more (Atkin et al, 2019).
Diabetes is a systemic disease that has been recently described as having reached epidemic proportions in modern society (Ferreira, 2020). Trophic disorders and infections are common complications in the feet of patients with advanced stages of the disease and are the main cause of amputation of the lower limb in those patients with diabetes (Ferreira, 2020). Diabetic foot complications lead to severe medical, social and economic consequences for the patients, while also posing a substantial public health problem (Piaggesi and Apelqvist, 2017). The threat of ulceration and amputation is higher in people suffering from diabetes compared to people without diabetes, and it is estimated that, somewhere in the world, every 20 seconds, an amputation is performed on an individual living with diabetes (Adiewere et al, 2018).
Foot ulceration is a common morbidity among diabetic complications as it is estimated that, annually, foot ulcers develop in 9.1 million to 26.1 million people with diabetes worldwide (International Diabetes Federation, 2015). More than 5% of people with diabetes have a history of foot ulceration and the cumulative lifetime incidence may be as high as 25% (Piaggesi and Apelqvist, 2017), while Armstrong et al (2017) suggest that between 19% and 34% of people with diabetes are likely to be affected by foot ulceration.
Clinicians involved in wound care traditionally have relied on habit, expert opinion and available product and treatment information (Cowan and Stechmiller, 2009). However, it is stated that wound management research improves patient care and clinical outcomes by standardising assessment, planning and implementation of treatment (World Union of Wound Healing Societies, 2020). It is generally agreed that relevant, good standard scientific evidence should be used to influence practice to help clinicians provide the best and most appropriate care for patients (Woodbury and Kuhnke, 2014). The implementation of evidencebased wound care concurs with better clinical outcomes for the patients and cost savings for the care systems and payers (Pacella et al, 2018).
Why TLC-NOSF dressings in the management of chronic wounds?
Recommendations on the appropriate treatment and care of people with specific diseases and conditions, should be based on the best available evidence, aiming to improve the quality of healthcare (Garbi, 2021). Two such guidelines were published in 2019 considering the management of DFUs. The International Working Group for Diabetic Foot (IWGDF) consider the use of technology lipido-colloid nano-oligosaccharide factor dressings (TLC- NOSF) in non-infected, neuroischaemic DFUs in order to enhance the wound-healing process (Rayman et al, 2019).
In addition, the National Institute for Health and Care Excellence (NICE) states that “evidence supports the case for adopting UrgoStart dressings to treat diabetic foot ulcers and venous leg ulcers in the NHS, because they are associated with increased wound healing compared with non-interactive dressings. UrgoStart dressings should therefore be considered as an option for people with diabetic foot ulcers or venous leg ulcers after any modifiable factors such as infection have been treated” (NICE, 2019).
Furthermore, in a recent systematic review, the authors concluded that: “All the evidence provided suggest that these dressings provide clinicians with an evidence-based option for the management of chronic wounds; that the TLC-NOSF dressings are beneficial in promoting the healing process, reducing healing times, enhancing patients’ health-related quality of life, and in allowing a more cost-effective procedure (Nair et al, 2021).
The potassium salt of sulfated oligosaccharides in the TLC-NOSF dressing has been shown to provide biological activities, including, reduction of matrix metalloproteases (MMPs), interaction with growth factors and restoring biological functions, thus promoting wound repair and shortening time to wound healing (Volkin et al, 1993; White et al, 2015). It has also been demonstrated that, when treating neuroischaemic DFUs with a TLC-NOSF dressing, transcutaneous oxygen pressure is improved (Lázaro-Martínez et al, 2020).
The aforementioned systematic review by Nair et al (2021) lists 21 publications of different levels, ranging from double-blind randomised controlled trials (RCT) to case reports, involving over 12,000 patients (Nair et al, 2021). Of main note are the RCTs, including the WHAT (Wound Healing Active Treatment) RCT, involving 117 patients presenting with VLUs, 27 centres which established superior wound area reduction (P=0.006) and better healing rates (P=0.029) with TLC-NOSF dressing compared with another MMP-inhibitor dressing (Collagen – oxidised regenerated cellulose) (Schmutz et al, 2008). The ‘Challenge’ study was conducted with 187 patients from 45 centres with venous leg ulcers, showed significant improvements in early wound area reduction (P=0.002) and in patients’ quality of life with TLC-NOSF dressings compared with non-interactive dressings (Meaume et al, 2012; 2017). The European double-blind RCT ‘Explorer’ study, performed in patients presenting with neuro-ischaemic DFUs and involving 240 patients from 43 centres, demonstrated a significantly higher wound closure rate (P=0.002) and a shorter time-to-closure (P=0.029) were achieved with the TLC- NOSF dressing in comparison to a non-interactive dressing (Edmonds et al, 2018; Lázaro-Martínez et al, 2019).
Lázaro-Martínez et al (2019) go further to suggest that “treating DFUs with TLC-NOSF dressing and good SoC (standard of care) results in higher wound closure rates than with a neutral dressing and the same good standard of care, whatever the duration and the location of the treated wounds. However, the earlier the TLC-NOSF dressing is initiated in DFU treatment, the greater the benefits”. Moreover, reduced healing times for leg ulcers, pressure injuries and DFUs treated with the TLC-NOSF dressings were described in current practice (real-life studies) from pooled analysis of the data from eight observational studies involving 10,220 patients (Münter et al, 2017).
New wound dressings are now presented, formed of a pad of poly-absorbent fibres coated with the TLC-NOSF healing matrix. The introduction of the poly-absorbent fibres can be used in the management of wounds both in the granulation stage of wound healing, as well as wounds at the debridement stage (Sigal et al, 2019). The clinical efficacy and safety profile of this new modality was demonstrated in two interventional, prospective, single-arm clinical trials (Sigal et al, 2019). The results from these two clinical studies showed that the investigated dressings are an effective, safe and simple treatment for the local management of chronic wounds at the different stages of healing and leading wound closure (Sigal et al, 2019).
A prospective, multicentre, observational study involving 1,140 patients in 130 centres with chronic wounds of various aetiologies were managed with the investigated polyabsorbent TLC-NOSF dressing, was conducted in Germany (Dissemond et al, 2020). After a mean duration of 56±34 days, by the final visit, 48.5% of wounds have healed and 44.8% improved. Similar results were reported notwithstanding of wound aetiology or proportions of sloughy and granulation tissue when the treatment with polyabsorbent TLC-NOSF dressings was initiated.
In view of the above results, the authors’ intention was to investigate the performance of the polyabsorbent TLC-NOSF dressings in patients with DFUs from Kuwait.
Design, results and assessment
This pilot open-label, mono-centric (Diabetic Foot Center, Farwaniya Hospital, Kuwait), single arm, non-interventional observational study, was conducted between February and October 2021. Patients were followed up in an outpatient setting for a maximum duration of 18 weeks (or until healing). Patients presenting with a DFU at the ‘sloughy’ or granulating stage were included, while DFUs presenting with active infection were not included in the evaluation. The primary endpoint was to assess the overall improvement of DFU following management with the polyabsorbent fibre TLC-NOSF dressing (UrgoStart Plus®, Urgo), with the secondary objective was to assess overall performance of the dressing (from the clinicians’ and patients’ point of view).
Decisions regarding diagnosis and therapy were made by the clinic staff and their usual therapeutic procedure was not influenced by the study. Clinical best practices were implemented according to the protocols of the Center. The Center Staff could discontinue the use of the evaluated dressing and any patient’s participation in the study at any point of the observation.
The relevant demographic information and medical history, as well as the following information were recorded at the initial visit: DFU diagnosed as neuropathic, ischaemic, neuro-ischaemic; offloading; wound location; presence of multiple wounds (in case of patients presenting with multiple eligible wounds, the wound considered by the team as the most suitable was selected for the study); wound recurrence and duration.
Demographic information and relevant medical history of the patients, wound characteristics (aetiology, wound duration, wound area, wound bed tissue and exudate level), previous and current wound treatment were recorded at the initial visit. Informed consent was also completed in the initial visit. Wound characteristics, wound healing progression and the occurrence of adverse events were assessed during the interim visits, performed on a regular basis. Outcomes related to the final assessment visit included treatment evaluation and healing progression in wound area reduction.
A total of nine patients were recruited but two patients did not complete the evaluation (one dropped out and one experienced a wound infection). The remaining seven participants were all male aged 61.8 ± 8.893 (51 to 76). All patients had diabetes mellitus type 2. One patient had a previous kidney transplant and three patients had lower-limb ischaemia. Three of the patients were utilising offloading devices. Figure 1 describes the wound types.
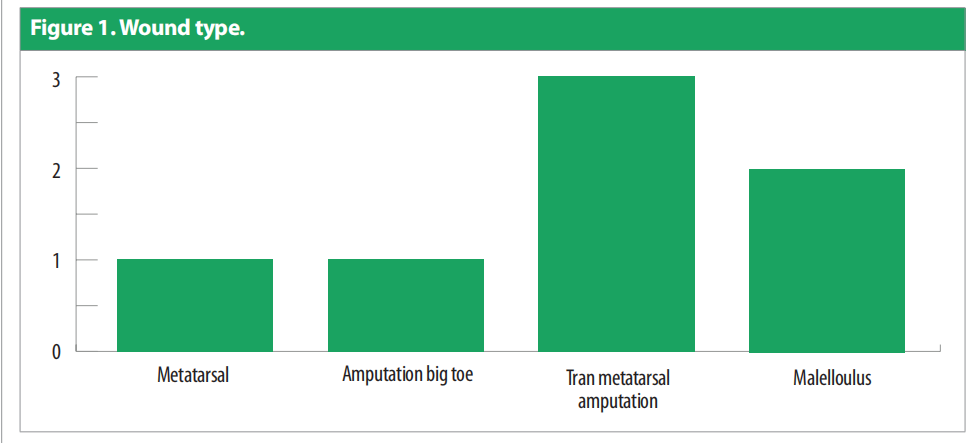
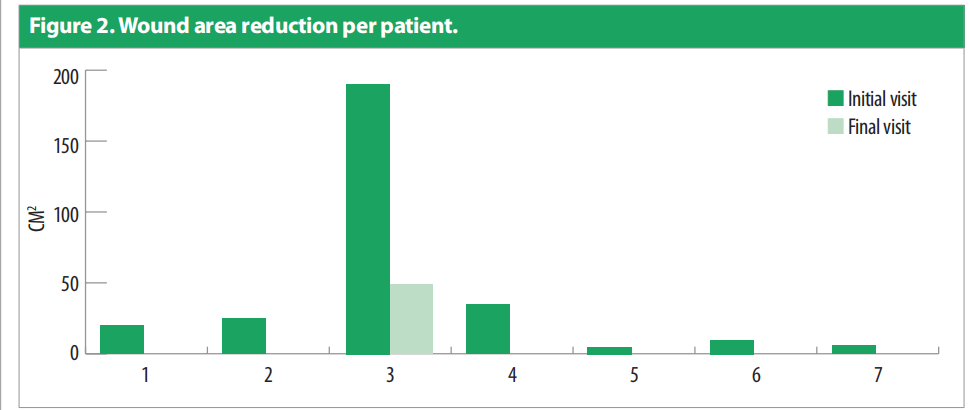
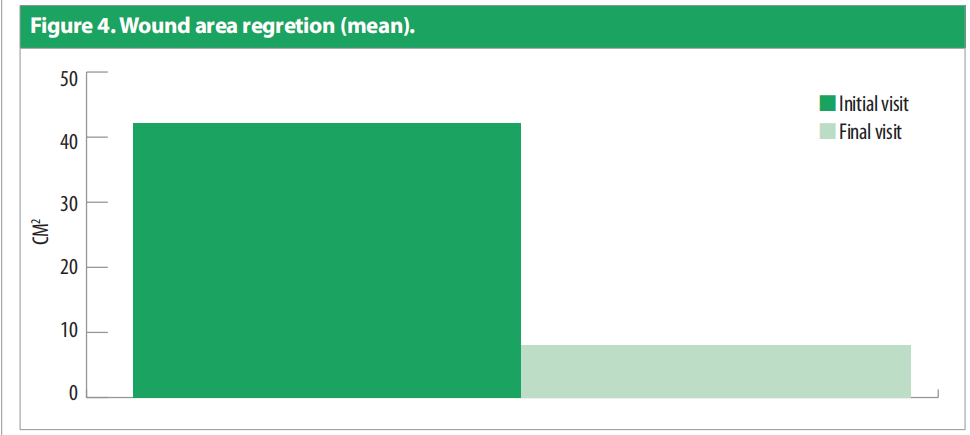
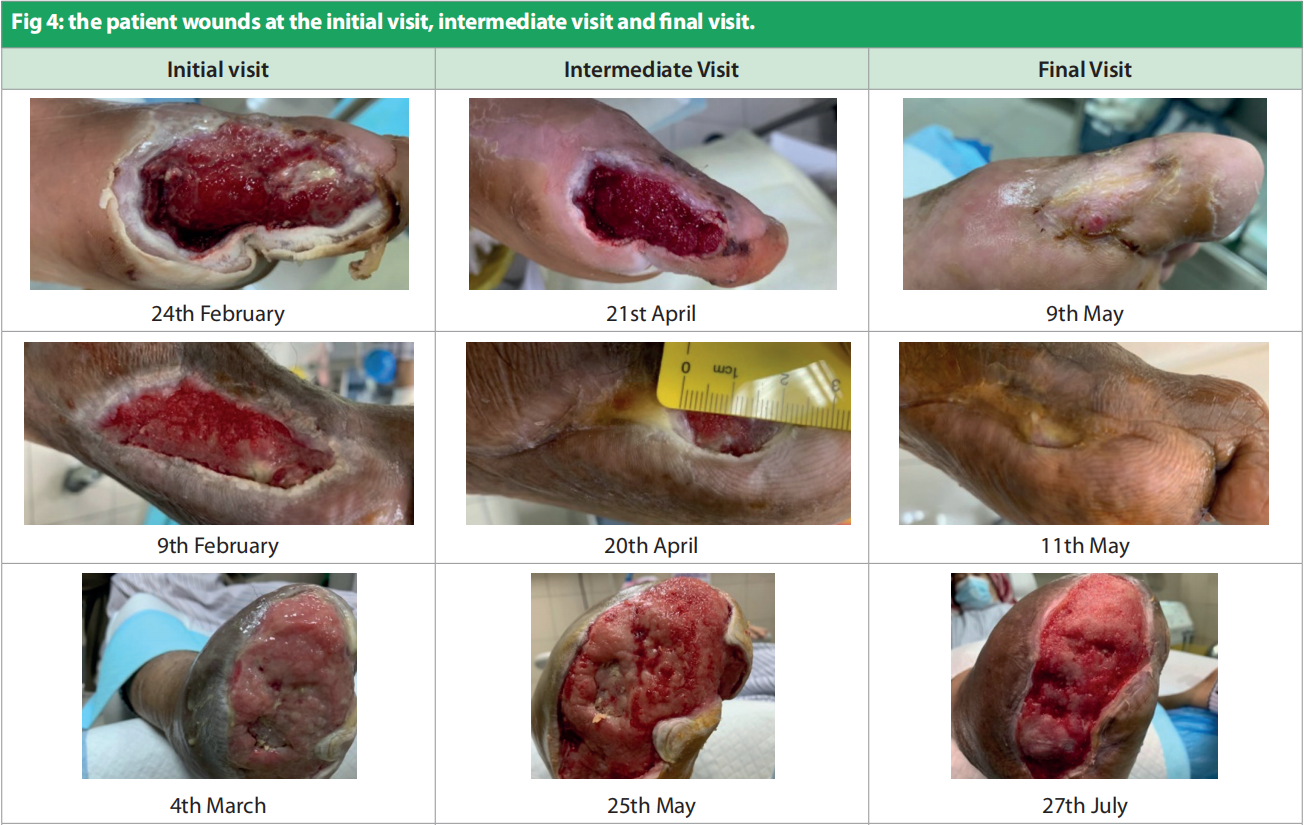
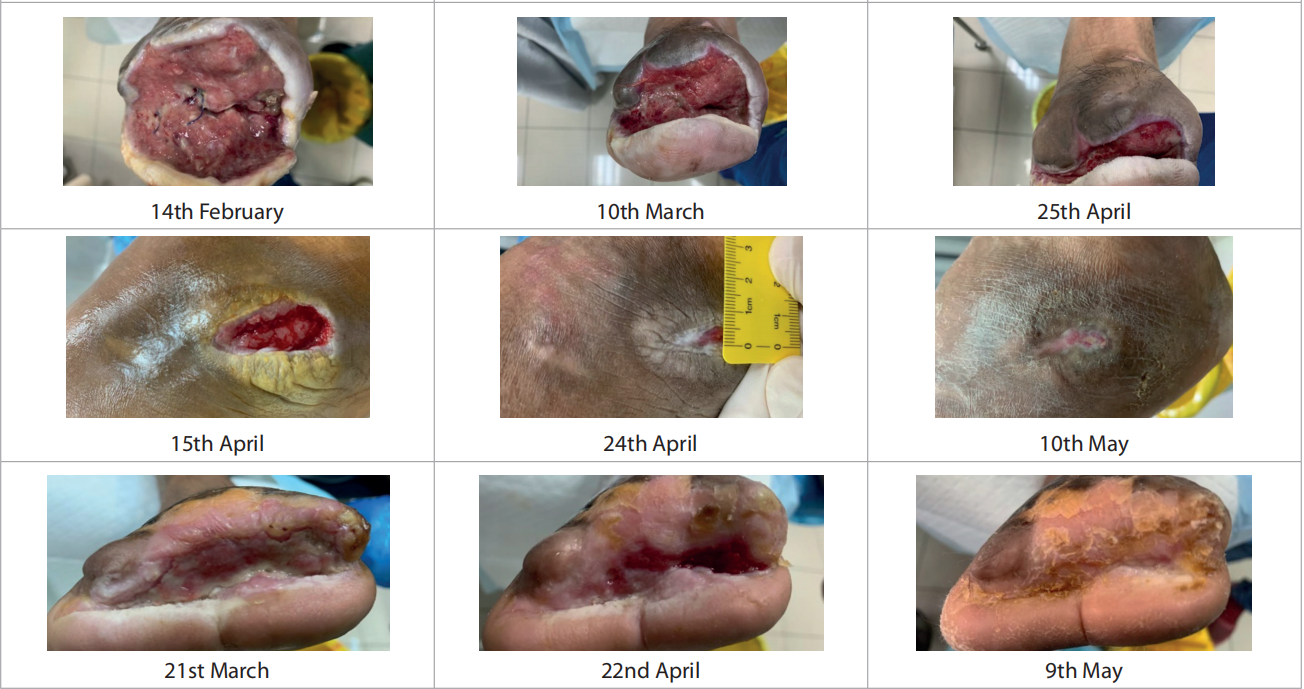
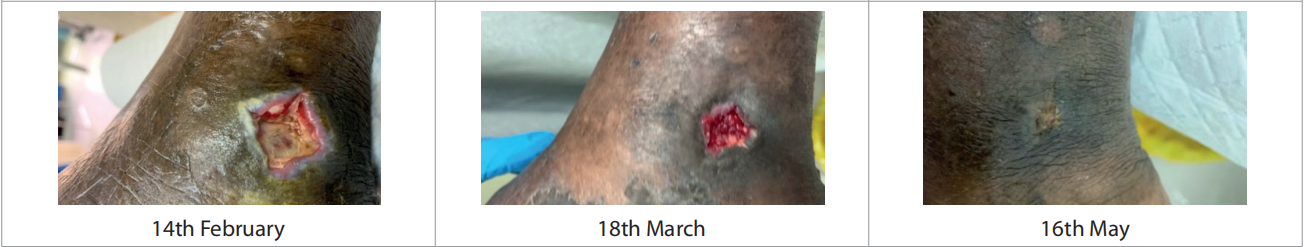
Only one patient had more than one wound. Duration of the wounds varied from 14 days to 180 days (55.428 ± 1.96). All patients were previously being managed by silver antimicrobial dressing apart from one for whom Omega-3 dressing was applied. Patients underwent debridement (sharp = 4, mechanical/ autolytic = 3) before starting the treatment. The patients were followed up between February and March 2021. Dressing changes were mostly conducted every 3 days. Initial wound area ranged from 4.5cm2 to 195cm2 [Figure 2], with a mean = 42.4cm2 , as compared to the final visit where the mean was 6.92cm2 [Figure 3]. All the wounds decreased in wound area, with five of the wounds (71.4%) completely healed. Figure 4 portrays the images of each wound at the initial, intermediate and final visits.
Discussion
The authors embarked on this pilot study on a limited cohort of patients to investigate the efficacy of the TLC-NOSF treatment range in advance of the planned project of a larger scale observational study. The decision to trial the polyabsorbent TLC-NOSF dressing in DFU patients from Kuwait was to evaluate if the results similar to those obtained in Germany (Dissemond et al, 2020) would be achieved. The German real-life observational study included 1,140 patients, with chronic wounds of various aetiologies (leg ulcers, diabetic foot ulcers, pressure ulcers, etc), which were treated with the investigated dressings in 130 centres, for a mean duration of 56±34 days.
By the final visit, 48.5% of wounds had healed and 44.8% had improved. The result of the evaluation was encouraging with the documented good results. These results will prompt further and more in-depth evaluation. Lessons learnt was that, in order to conduct a larger scale trial, the set-up, collection of data and analyses of the results need to be conducted in a better, more systematic manner. In the pursuing proposed study, the clinicians will gather data in a more uniform and professional manner by employing the services of a contract research organisation (CRO) to assist in the planning, setup, and day-to-day execution and management and analyses of the clinical data, in order to be able to present the study in the proficient manner expected.
Conclusion
Pilot studies are referred to as small scale scale versions, or a trial run, in preparation for a major study as pre-testing or trying out of a particular research intervention, mainly to assess the feasibility and likelihood of success (Van Teijlingen and Hundley, 2001). Copious evidence is available regarding the management of chronic wounds with the TLC-NOSF treatment range, which, however, was mainly conducted in Europe. “Replicability refers to a different team arriving at the same results using the original author’s artifacts” (NASEM, 2019). In view of this, the authors decided to do an initial evaluation of the TLC-NOSF treatment range in DFU patients from Kuwait. The initial results were very encouraging to conduct further investigations.
It was noted, however, that it might be beneficial if the trial might be conducted using a protocol that includes an antimicrobial and desloughing dressing prior to initialising the TLC-NOSF dressings — it is suggested that infections occur in up to 58% of patients presenting with a new foot ulcer (Del Core et al, 2018). A real-life observational study was conducted by a German team headed by Dissemond (2020) including 2,270 patients with acute and chronic wounds of various aetiologies that were treated with a poly-absorbent silver dressing with technology lipido-colloid with silver ions, TLC-Ag (UrgoClean Ag®, Urgo) dressing for a mean duration of 22±13 days. All clinical signs of local infection and the diagnosed wound infections were substantially reduced at 2 weeks after the treatment initiation, while all wound infection parameters continued to reduce until the last visit. The suggested new protocol will include the procedure of utilisation of this antimicrobial dressing to remove any overt or covert infection prior to starting the TLC-NOSF treatment.
Conflict of interest
There was no conflict of interest.
References
1. Adiewere P, Gillis RB, Jiwani SI et al (2018) A systematic review and meta-analysis of patient education in preventing and reducing the incidence or recurrence of adult diabetes foot ulcers (DFU). Heliyon 4(5): e00614
2. Armstrong DG, Boulton AJ, Bus SA (2017) Diabetic foot ulcers and their recurrence. N Engl J Med 376(24): 2367–75
3. Atkin L, Bućko Z, Montero EC et al (2019) Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care 28(Sup3a): S1–50
4. Cowan LJ, Stechmiller J (2009) Prevalence of wet-to-dry dressings in wound care. Adv Skin Wound Care 22(12): 567–73
5. Del Core MA, Ahn J, Lewis III RB et al (2018) The evaluation and treatment of diabetic foot ulcers and diabetic foot infections. Foot Ankle Orthopaedics 3(3): 1–11
6. Dissemond J, Lützkendorf S, Dietlein M, et al (2020) Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care 29(6): 350–61
7. Dissemond J, Dietlein M, Neßeler I et al (2020) Use of a TLC-Ag dressing on 2270 patients with wounds at risk or with signs of local infection: an observational study. J Wound Care 29(3):162–73
8. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM et al (2018) Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol 6(3):186–96
9. Ferreira RC. Diabetic Foot (2020) Part 1: Ulcers and Infections. Rev Bras Ortop (Sao Paulo) 55(4): 389–96
10. Garbi M (2021) National Institute for Health and Care Excellence clinical guidelines development principles and processes. Heart heartjnl-2020-318661
11. Greeley PW (1953) Plastic surgical closure of chronic open wounds of the leg. Ind Med Surg 22(1): 22–3
12. International Diabetes Federation (2015) IDF Diabetes Atlas (7th edn.) Brussels: International Diabetes Federation
13. Kyaw B, Järbrink K, Martinengo L et al (2018) Need for improved definition of “chronic wounds” in clinical studies. Acta Derm Venereol 98(1):157–8
14. Lázaro-Martínez JL, Edmonds M, Rayman G et al (2019) Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer. J Wound Care 28(6): 358–67
15. Lázaro-Martínez JL, García-Madrid M, García-Alamino JM et al (2020) Increasing transcutaneous oxygen pressure in patients with neuroischemic diabetic foot ulcers treated with a sucrose octasulfate dressing: a pilot study. Int J Low Extrem Wounds 1534734620952244
16. Meaume S, Truchetet F, Cambazard F et al (2012) A randomized, controlled, double-blind prospective trial with a lipido-colloid technology-nanooligosaccharide factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen 20(4): 500–11
17. Meaume S, Dompmartin A, Lok C et al (2017) Quality of life in patients with leg ulcers: results from CHALLENGE, a double-blind randomised controlled trial. J Wound Care 26(7): 368–79
18. Münter KC, Meaume S, Augustin M et al (2017) The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care 26(Sup2): S4–S15
19. Nair H, Venkateshwaran N, Seetharaman S et al (2021) Benefits of sucrose octasulfate (TLC-NOSF) dressings in the treatment of chronic wounds: a systematic review. J Wound Care 30(Sup4): S42–52
20. National Academies of Sciences, Engineering, and Medicine, Policy and Global Affairs, Committee and Science, Engineering, Medicine, and Public Policy et al (2019) Reproducibility and Replicability in Science. Washington, DC: The National Academies Press
21. NICE (2019) Medical technologies guidance [MTG42]. UrgoStart for Treating Diabetic Foot Ulcers and Leg Ulcers. Medical Technologies Guidance [MTG42]. Available at: https://bit.ly/3GERaA2 (accessed 08.02.2022)
22. Pacella RE, Tulleners R, Cheng Q et al (2018) Solutions to the chronic wounds problem in Australia: a call to action. Wound Practice & Research 26(2): 84–98
23. Piaggesi A, Apelqvist J (2017) The diabetic foot syndrome today: a pandemic uprise. In: Piaggesi A, Apelqvist J (eds.) The Diabetic Foot Syndrome. New York: Karger Medical and Scientific Publishers; 16: 1–18
24. Rayman G, Vas P, Dhatariya K et al (2020) Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 36 (Suppl 1): e3283
25. Schmutz JL, Meaume S, Fays S et al (2008) Evaluation of the nanooligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int Wound J 5(2):172–82
26. Sigal ML, Addala A, Maillard H et al (2019) Evaluation of TLC-NOSF dressing with poly-absorbent fibres in exuding leg ulcers: two multicentric, single-arm, prospective, open-label clinical trials. J Wound Care 28(3): 164–75
27. Van Teijlingen E, Hundley V (2001) The Importance of Pilot studies. Social Research Update. University of Surrey 35: 1–4. Available at: https://bit.ly/3oxhOVn (accessed 08.02.2022)
28. Volkin DB, Verticelli AM, Marfia KE et al (1993) Sucralfate and soluble sucrose octasulfate bind and stabilize acidic fibroblast growth factor. Biochim Biophys Acta 1203(1): 18–26
29. Woodbury MG, Kuhnke JL (2014) Evidence-based practice vs. evidence-informed practice: what’s the difference. Wound Care Canada 12(1): 18–21
30. White R, Cowan T, Glover D (2011) Supporting evidencebased practice: a clinical review of TLC technology. J Wound Care 20(Sup1): 1–36
31. World Health Organization (2022) Diabetes Registry – Kuwait. Available at: https://bit.ly/34BLGZz (accessed 08.02.2022)
32. World Union of Wound Healing Societies (2020) Evidence in Wound Care. London: Wounds International. Available at: https://www.woundsinternational.com/ resources/details/evidence-wound-care (accessed 08.02.2022)
This article is excerpted from the Wounds International 2022 | Vol 8 Issue 1 by Wound World.



