Systemic sclerosis (SSc) is an autoimmune disease characterized by microvascular damage, obliteration, and innate and adaptive immunity dysregulation leading to tissue fibrosis. Vascular dysfunction and endothelial damage play a central role in the pathogenesis of SSc [1] and digital ischemia is the most characteristic clinical manifestation. Whereas microvascular involvement is considered to be the hallmark of SSc,several studies have shown the increased prevalence of macrovascular arterial disease and the presence of early and accelerated atherosclerosis, particularly in the lower limbs [2,3]. Macrovascular arterial impairment in SSc may worsen the digital ischemia related to microvascular disease, leading to an increased risk of ischemic ulcers in the lower and upper limbs [3].
Routine tests to detect and diagnose peripheral arterial disease (PAD) include physical examination, hemodynamic measures, such as ankle brachial pressure index (ABI) and toe-brachial pressure index (TBI), and imaging assessment by lower limb duplex ultrasonography (US) or angiography [2, 4]. In our experience, the palpation of foot pulses, recommended in daily practice, is not reliable in SSc patients due to skin sclerosis or leg edema.
ABI is calculated by dividing the ankle systolic pressure by the brachial systolic pressure. Measuring ABI is internationally recommended to assess peripheral vascular status in patients at risk of PAD [5]. Normal values are between 0.9 and 1.3. An ABI below 0.9 indicates the presence of PAD with high sensitivity and specificity [6]. Decreased sensitivity and specificity of ABI measurement for PAD has been demonstrated in the elderly and in patients presenting diabetes mellitus or chronic renal failure. In these cases, ABI may be falsely normal or above 1.3 because of the loss of ankle artery compressibility [7]. In such situations, measuring toe pressure and toe-brachial index (TBI) has been shown to be an indicator of proximal or distal peripheral arterial impairment as the measure of toe pressure is never affected by arterial stiffness [8]. TBI is the ratio between the systolic toe pressure and the systolic brachial pressure, with normal values greater than 0.70 or 0.75, depending on recommendations [9]. TBI has been shown to have greater clinical efficacy in the diagnosis of PAD than ABI but is less accessible in routine practice.
The primary aim of this prospective study was to assess the frequency of lower limb arterial impairment in SSc patients by measuring ABI and TBI, complemented if necessary, by lower limb duplex US imaging. In addition, we determined clinical characteristics that are associated with abnormal TBI at inclusion and during follow-up.
Patients and methods
This prospective, observational, cohort study, using routinely collected data, was conducted in compliance with the Declaration of Helsinki, between 2015 and 2019. According to French law, formal Ethics Committee approval was not required for this type of study. Consecutive SSc patients were recruited between 2015 and 2016 from the Tenon Hospital Dermatology Department and the Internal Medicine Department of Saint Antoine Hospital, Assistance Publique Hôpitaux de Paris (AP-HP), Paris, France, then followed up until 2019. All patients included fulfilled the 2013 ACR/EULAR criteria for SSc [1] and attended the hospital for a routine follow-up visit. Oral consent was collected from all patients included.
Clinical assessment
Patients’ medical histories and treatments were assessed, and they underwent a complete physical examination. Patients were systematically evaluated for the presence of arterial hypertension and pulmonary, cardiac, esophageal, and renal involvoment.
During follow-up, major adverse events (MAE) were defined as all-cause mortality, non-fatal stroke, non-fatal myocardial infarction, and lower limb ischemic manifestations.
Measurement of arterial pressure and duplex US imaging Examinations were performed, once at inclusion, in a room with a temperature over 24°C and patients’ feet were warmed to obtain a cutaneous temperature of 28°C, verified by infrared cutaneous thermometer, to avoid any impact of a possible phenomenon of Raynaud on the measurement [10]. Measurements were performed after a 10-min rest in supine position.
Ankle systolic pressure in the dorsalis pedis and posterior tibial arteries at the ankle was measured using an 8-MHz Doppler probe and a cuff (12 cm) around the lower leg just above the ankle.
For toe blood pressure, a cuff with a width depending on the diameter of the hallux (1.5-, 2.5-, or 3.3-cm disposable cuffs, Hokanson, Bellevue, Washington) was wrapped around the base of the toe. Two standard laser Doppler (LD) probes (PF 408, Perimed, Järfälla, Sweden) connected to the LD instrument (Periflux 4001, Perimed, Järfälla, Sweden) were attached to the apex of the big toe with double-sided adhesion tape with a notch for the probe (Double-Stick Discs, 3M Health Care, Borken, Germany) and a probe holder (PF 104, Perimed Järfälla, Sweden). The cuff was rapidly inflated to a pressure at least 30 mmHg higher than that at which the pulsations in the LD 0.03 signal disappeared. Initially, the LD showed an increase in flux, after which the signal declined to the baseline. After the LD signal had reached a stable baseline (after approximately 3 s), the cuff was slowly deflated with a speed of approximately 3 mmHg/s. The systolic pressure was defined as the pressure at which the (pulsatile) signal reappeared or rose gradually from the baseline.
ABI and TBI were calculated as the highest ankle (dorsalis pedis or posterior tibial artery) and toe systolic pressures divided by the highest brachial systolic pressure, respectively. We performed measurement on both legs and considered the worst result.
For patients with abnormal TBI and/or ABI, a color duplex US was conducted on the lower limbs. Peripheral arterial disease was defined as the presence of parietal thickening, and stenosis was defined as the presence of one > 50% stenosis, in any studied artery.
Classification of lower limb hemodynamic vascular involvement
Consistent with current guidelines [11], an ABI value <0.9 indicated the presence of a PAD with hemodynamic stenosis, an ABI ≥0.9 and ≤1.3 was considered as normal and an ABI >1.3 as evidence of loss of arterial compressibility. Although there is no consensus on the pathological limit, TBI values <0.75 were considered to reflect the presence of peripheral lower limb arterial involvement as validated in a casecontrol study on diabetes patients [12]. A low ABI value always implies a decreased TBI value as the latter is measured on more distal arteries. For normal ABI values, TBI may be either normal in absence of peripheral arterial involvement or decreased in cases of falsely normal ABI related to a partial loss of artery compressibility or in cases of isolated distal arterial involvement (below the ankle).
We classified patients as follows, according to clinical examination, using ABI and TBI:
normal hemodynamic status (normal ABI and TBI);
lower limb arterial impairment (low TBI):
isolated microvascular impairment (low TBI associated with both normal or high ABI and absence of arterial stenosis on lower limb duplex US);
macrovascular impairment (TBI and ABI both low; or low TBI with arterial stenosis on lower limb duplex US, regardless of ABI);
isolated arterial vascular stiffness (isolated ABI >1.3 with normal TBI).
Statistical analysis
Quantitative variables were reported as median and interquartile range (IQR), qualitative ones by number and percentage. Univariate comparisons between cases and controls used Mann-Whitney tests, for quantitative variables, and Pearson’s χ2 tests or Fisher’s exact tests, for qualitative variables. Time to event analysis was done using the KaplanMeier method and groups were compared by the log-rank test. The multivariate logistic regression model was used to identify parameters independently associated with low TBI with astepwise procedure based on Akaike information criterion (AIC). Variables with p<0.1 in the univariate analysis were included in the multivariate logistic regression model. Age, gender, and digital ulcers were forced into the model for adjustments.
Two-tailed “p” less than 0.05 was considered statistically significant. Statistical procedures were performed using R version 3.1.3 statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of patients
Eighty-six patients were included between September 2015 and September 2016; 74.4% of them presenting limited cutaneous systemic sclerosis (Table 1). The median follow-up was 29.3 months, IQR: [24.70–31.8], and 6 patients out of 86 were lost to follow-up.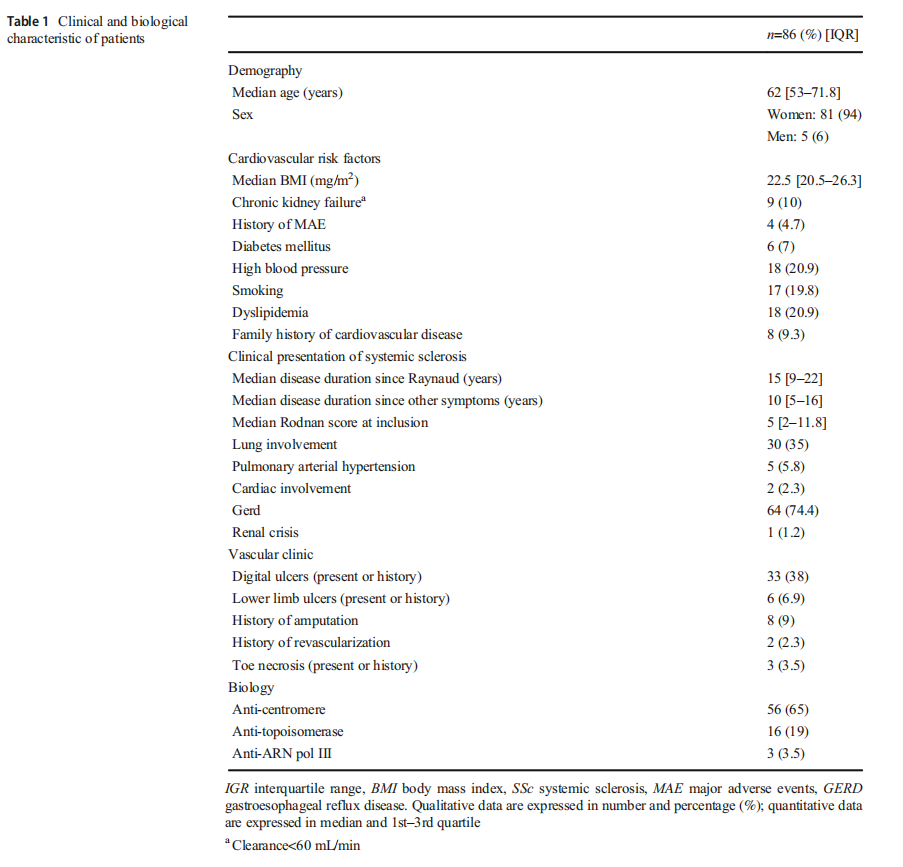
Clinical manifestations of lower limb peripheral arterial involvement
All patients presented Raynaud phenomenon, 66 (76.7%) had no other clinical vascular manifestations (i.e., abnormal pulse, vascular claudication, rest pain), 11 (12%) had isolated abnormal pulses (either posterior tibialis or dorsalis pedis) at the ankle, without functional signs (vascular claudication or rest pain), and 7 (8%) had both abnormal pulses at the ankle and functional signs. Vascular claudication was present in 6 patients and rest pain in 2 patients.
Normal hemodynamic status is rare during SSc
Only 21 (24.4%) SSc patients had no lower limb hemodynamic arterial involvement characterized by both normal ABI comprised between 0.9 and 1.3 and normal TBI>0.75 (Fig. 1).
Most SSc patients exhibit decreased distal arterial perfusion in the lower limbs Considering all cases with a TBI<0.75, impairment of distal arterial perfusion was present in 53 (61.6 %) patients (Fig. 1).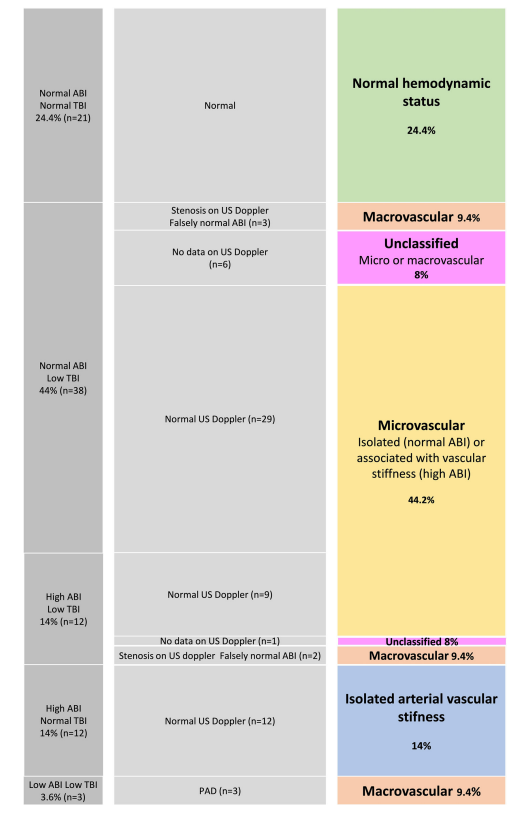

Isolated microvascular impairment
Thirty-eight (44%) had isolated distal perfusion impairment defined as a TBI<0.75, associated with a normal or high ABI and a normal duplex US. Among them, 9 had a high ABI reflecting microvascular impairment associated with arterial stiffness.
Peripheral arterial disease
PAD was present in 8 (9.3%) patients. Among them, an ABI<0.9 and a TBI<0.75 reflecting an arterial stenosis above the ankle were found in 3 patients. A normal or high ABI associated with a low TBI and a significant arterial stenosis above the ankle on duplex US was found in 5 other patients.
Among patients with a low TBI, a duplex US could not be obtained in 7 patients who therefore could not be classified as patients presenting PAD or isolated microvascular
High prevalence of isolated arterial vascular stiffness Elevated ABI with normal TBI was found in 12 (14%) patients, reflecting isolated arterial vascular stiffness. For these patients, all duplex USs we obtained were normal (Fig. 1).
Abnormal TBI is associated with clinical manifestations
We considered a TBI<0.75 for univariate and multivariate analyses. In univariate analysis, the low TBI group had more lower limb sclerosis (OR=7.44, p=0.02) and were treated more commonly with statins (OR=3.59, p=0.04), calcium channel blockers (OR=4.17, p=0.01), and iloprost (OR=3.22, p=0.04). Multivariate analysis did not reveal any significant association between the presence of an abnormal TBI and clinical or biological SSc characteristics.
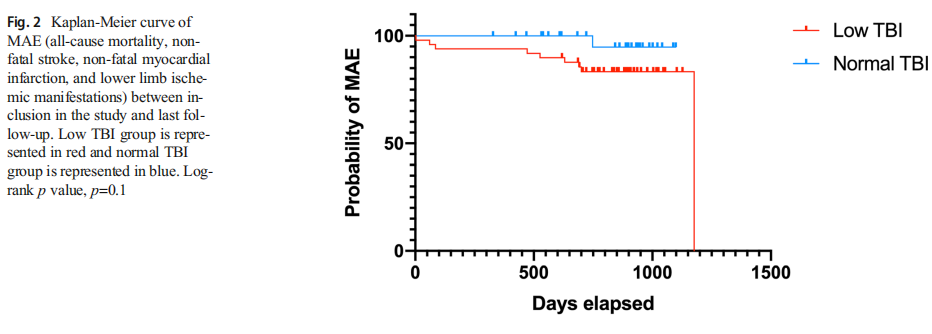
Among 46 patients who had both low TBI and an available duplex US, parietal vascular infiltration was observed in 26 patients and significant stenosis only in 6 patients. The mean follow-up duration was similar in both groups (29 [22.75–31] months in the low-TBI group and 28 [23–31] months in the normal-TBI group, p=0.13) and mean time to MAE from diagnosis, defined by the beginning of Raynaud phenomenon was 18.2 [8.6–35.9] years. During follow-up, there was a trend towards association of low TBI with more MAE (allcause mortality, non-fatal stroke, non-fatal myocardial infarction, and lower limb ischemic manifestations) than normal TBI, though this trend was not statistically significant (p=0.08) (Table 2)(Fig. 2).
Discussion
In this study, 76% of SSc patients presented a lower limb peripheral hypoperfusion and 28% an elevated ABI in line with arterial stiffness. Moreover, among the 68% of SSc patient who had a normal ABI, more than half also had a low TBI, reflecting either isolated microvascular disease or falsely normal ABI due to the frequent presence of vascular stiffness. Low TBI at inclusion was associated with MAE during follow-up, although not significantly, probably due to lack of power. The absence of significance between low TBI and vascular clinical manifestations is potentially related to the lack of power.
Our study evaluated the frequency of arterial stiffness during SSc (ABI>1.3), and assessed peripheral perfusion by measuring both ABI and TBI. The high frequency of arterial stiffness seems consistent with the pathophysiology of the disease, which associates early vascular and inflammatory alterations, and progressive vessel wall thickening. The association of a low TBI with a normal ABI is higher in our study (44%) than in the general population or in populations suspected of PAD, where it was estimated to be between 9 and 27% [8]. In SSc patients, ABI measurement alone is not a valid tool to detect early peripheral hypoperfusion for two reasons. First, vascular stiffness is frequent, and ABI may be falsely normal in this case, as has already been reported in diabetic patients [7]. Studies comparing mean or median ABI between SSc and controls may therefore underestimate the prevalence of PAD in this population. Second, SSc microvascular disease may have an additive effect on proximal arterial impairment, worsening the peripheral ischemia. In view of our results, ABI alone should not be used to test for lower limb arterial disease in SSc patients, but in combination with TBI, or with duplex US if TBI is not available [9].
Macrovascular involvement during SSc is characterized by the formation of a neointima leading to intimal hyperplasia. The relative risk of lower limb arterial involvement during SSc is estimated between 4.4 and 9.2. Moreover, a recent systematic review showed that SSc was an independent risk factor of coronary heart disease (OR between 1.7 and 3.3) [13].
Our study has some limitations: first, despite a reasonable population cohort, we were not able to reach significance concerning the association between TBI and MAE because of a short follow-up duration; second as discussed below, there is no consensual TBI value in the literature. Finally, this study was an observational study, thus we could not obtain US doppler from all patients with normal TBI/ABI and from a control population.
There is no consensus on the pathological limit of TBI. We chose 0.75 as it had been validated in a case-control study on diabetes patients [12]. Some guidelines proposed a TBI threshold of 0.7, but did not include any references offering strong support for this limit [9].
The prevalence of low ABI<0.9 in our population was comparable to that reported in the general population: we observed it in 3.49% (95% CI 0.00% to 7.37%) of patients, compared to 4.3% (95% CI 3.1% to 5.5%) in the general population defined from the National Health and Nutrition Examination Survey, 1999–2000; this population was characterized by a mean age of 56.5 years old, 48% were male and 76.9% had cardiovascular risk factor [9]. Our results are in accordance with the studies of Bartoli et al. [14] and Kaloudi et al. [15], who reported a prevalence of low ABI in SSc patient of 5.3% and 4.5%, respectively. Only Ho et al. [16] found a greater prevalence of low ABI, at 17%.
Monitoring of traditional cardiovascular risk factors is required in cases of micro- or macrovascular impairment, such as the implementation of preventive measures to avoid traumatic wounds on the feet. Although it did not reach significance, a low TBI seems to be associated with overall prognosis in SSc patients, highlighting the fact that these patients should be carefully managed regarding cardiovascular disease.
In this prospective study, we showed that 76% of SSc patients had hemodynamic arterial lower limb abnormalities and that 28% had vascular stiffness, illustrated by an elevated ABI, which in any case appears to be a poor indicator when screening for PAD during SSc. Measuring toe pressure seems essential in diagnosing lower limb PAD during SSc, as it is recommended in diabetes mellitus and chronic kidney failure, two factors also associated with arterial stiffness.
Compliance with ethical standards Ethics approval
Ethics committee approval was not required for this type of study
Consent to participate
Oral consent was collected from all patients
Disclosures None
References
1. Van Den Hoogen F, Khanna D, Fransen J et al (2013) 2013 classification criteria for systemic sclerosis: an american college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 65:2737–2747. https://doi.org/10.1002/ art.38098
2. Veale DJ, Collidge TA, Belch JJ (1995) Increased prevalence of symptomatic macrovascular disease in systemic sclerosis. Ann Rheum Dis 54:853–855
3. Bohelay G, Blaise S, Levy P, Claeys A, Baudot N, Cuny JF, Maillard H, Granel-Brocard F, Boyé T, Lok C, Bénéton N, Francès C, Senet P (2018) Lower-limb ulcers in systemic sclerosis: a multicentre retrospective case-control study. Acta Derm Venereol 98:677–682. https://doi.org/10.2340/00015555-2939
4. Muro Y, Sugiura K, Morita Y, Tomita Y (2009) An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 27:26–31
5. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, TreatJacobson D, Walsh ME (2017) 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American college of cardiology/American Heart Association task force on clinical practice guidelines. Circulation 135:e686–e725
6. Dachun X, Jue L, Liling Z et al (2010) Sensitivity and specificity of the ankle–brachial index to diagnose peripheral artery disease: a structured review. Vasc Med 15:361–369. https://doi.org/10.1177/ 1358863X10378376
7. Potier L, Abi Khalil C, Mohammedi K, Roussel R (2011) Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg 41:110–116. https://doi.org/10.1016/j.ejvs.2010.09.020
8. Høyer C, Sandermann J, Petersen LJ (2013) The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg 58:231–238.https://doi.org/10.1016/j.jvs.2013.03.044
9. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR (2007) Inter-society consensus for the management of peripheral arterial disease (TASC II). Int Angiol 26:82–157. https://doi.org/10.1016/j.jvs.2006.12.037
10. Tehan PE, Santos D, Chuter VH (2016) A systematic review of the sensitivity and specificity of the toe-brachial index for detecting peripheral artery disease. Vasc Med 21:1–8. https://doi.org/10. 1177/1358863X16645854
11. Gerhard-Herman MD, Gornik HL, Barrett C et al (2017) 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: executive summary. American Heart Association, Inc
12. Williams D, Harding K, Price P (2005) An evaluation of the efficacy of methods used in screening for lower limb arterial disease in diabetes mellitus. Diabetes Care 28:2206–2210. https://doi.org/10.2337/diacare.28.9.2206
13. Ali H, Ng KR, Low AH (2015) A qualitative systematic review of the prevalence of coronary artery disease in systemic sclerosis. Int J RheumDis 18:276–286
14. Bartoli F, Angotti C, Fatini C, Conforti ML, Guiducci S, Blagojevic J, Melchiorre D, Fiori G, Generini S, Damjanov N, Rednic S, Pignone A, Castellani S, Abbate R, Matucci Cerinic M (2007) Angiotensin-converting enzyme I/D polymorphism and macrovascular disease in systemic sclerosis. Rheumatology 46: 772–775. https://doi.org/10.1093/rheumatology/kel433
15. Kaloudi O, Basta G, Perfetto F, Bartoli F, del Rosso A, Miniati I,Conforti ML, Generini S, Guiducci S, Abbate R, Pignone A, Castellani S, Livi R, de Caterina R, Matucci-Cerinic M (2007) Circulating levels of Ne-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology 46:412–416. https://doi.org/10. 1093/rheumatology/kel076
16. Ho M, Veale D, Eastmond C, Nuki G, Belch J (2000) Macrovascular disease and systemic sclerosis. Ann Rheum Dis 59:39–43
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is by wound world,excerpted from the Clinical Rheumatology.


