1.Introduction
Chronic venous disease (CVD) is one of the most common peripheral vascular diseases, with varicose veins of lower extremities as the main manifestation [1]. The incidence rate is about 25-40% in the adult population worldwide [2]. Its clinical manifestations such as swelling, pigmentation, lipid sclerosis, and ulceration seriously affffect patients’ physical and mental health and greatly increase the social and economic burden [3, 4].
New strategies such as laser, radiofrequency ablation, cyanoacrylate glue, and other intracavitary minimally invasive techniques have improved varicose vein treatment in the lower extremities in recent years [5, 6]. However, intracavitary treatment still fails to effffectively overcome the limitations of its application scope and high long-term recurrence rate compared with exfoliation surgery [7, 8]. Stripping the great saphenous vein (GSV) has long been considered the operating standard for varicose vein treatment of the lower extremities, obtaining satisfactory longterm clinical effffects [9, 10]. Signifificantly, the emergence of a variety of improved technologies allows for GSV exfoliation to be more minimally invasive and safe [11]. In this study, we analyzed the clinical data of patients with great saphenous vein avulsion in the fifirst hospital of Hebei Medical University and compared the application effffect. This study could provide important implications for the future application of modifified above-knee surgery.
2.Methods and Materials
2.1. Four hundred twenty-six patients with GSV exfoliation were selected from the First Hospital of Hebei Medical University from May 2016 to May 2018.
The inclusion criteria were as follows: (1) age 18-75 years old without gender limitation; (2) clinical, etiologic, anatomic, and pathophysiologic (CEAP): grade C2-C5; (3) GSV reflflux time in standing position > 0:5 s and diameter of proximal saphenous vein > 8 mm [4]; (4) discomfort symptoms related to GSV reflflux, such as heavy and pain.
Exclusion criteria are listed as follows: (1) a history of surgery; (2) venous thromboembolism; (3) post deep vein thrombosis syndrome; (4) severe deep vein reflflux; (5) Fontaine stage II-IV of arteriosclerosis obliterans; (6) organ dysfunction intolerant to surgery; (7) sclerotic allergy. The Medical Ethics Committee of The First Hospital of Hebei Medical University (20200507) approved this study.
2.2. Diagnostic Method. In total, 426 patients with confifirmed varicose vein defifinition following recommendations of the CEAP classifification and the recently updated venous terminology of the International Committee of the AVF [12] and imaging (Doppler ultrasonography) examinations were enrolled into the prospectively gathered data.
Based on the surgery method, the patients were divided into modifified above-knee (N = 213) and conventional surgery groups (N = 213). The CVD in the lower extremity was evaluated by CEAP grading [13], venous clinical severity score (VCSS) [14], and chronic venous insuffiffifficiency questionnaire (CIVIQ-14) [15]. CIVIQ-14 score was calculated using the formula: CIVIQ‐14 = ðfinal score − 14/56Þ × 100. The diameter of proximal GSV from the junction of the saphenous femoral vein was 0.5 cm. The operation time was from skin incision to completion of compression bandage with an elastic bandage. Blood loss volume was calculated based on the area of blood loss, i.e., 1 cm2 as 1 ml. The discharge standard included no fever, pain, bleeding, or exudation. Postoperative recurrence was varicose vein recurrence in the treatment area one month later. Completely stripping the GSV was considered as a successful surgery. Disappearing of symptoms or an improvement of symptoms one month postoperation was considered as clinical success, and no varicose vein was visible to the naked eye. The injury of the saphenous nerve was presented as discomfort of pain and numbness in the inner side of the leg postoperation. According to the degree of injury, mild injury was supposed when the surgery did not affffect walking. Otherwise, it was supposed as severe injury.
Surgery Methods. All operations on the study and control groups were performed by the same doctor with the same level of clinical experience under local or neuraxial anesthesia. Under ultrasound guidance before operation (Figure 1), varicose veins were drawn, and the positions needing treatment were marked. The anesthesia swelling flfluid consisted of 500 ml normal saline, 25 ml 2% lidocaine, 1 ml 0.1% adrenaline, and 20 ml 5% sodium bicarbonate. In the supine position, GSV was exposed at the mark of the inguinal ligament, branches were ligated, cut at a distance of 0.5 cm from the femoral vein, and the proximal part of GSV was ligated. In the conventional surgery group, the beginning of GSV was exposed at the medial malleolus, the proximal portion of GSV was inserted along the line from the groin, and the GSV was removed during the whole course of inversion. In modifified above-knee surgery, the great saphenous vein stripping catheter (Vein Harvest, Hangzhou Tiancheng Pharmaceutical Co., Ltd.) was placed at the groin incision, and the outer sleeve was peeled to the knee joint where it was cut offff. Under the ultrasound guidance, the anesthetic swelling liquid was injected around GSV. Residual branch varicose veins < 4 mm were treated with 1% polycicol foam (German Hameln pharmaceuticals GmbH) for sclerotherapy, and residual branch varicose veins ≥ 4 mm were treated by dot-based stripping. No additional surgical strategies such as a groin patch were used postoperation, the adhesive elastic bandage was used after applying pressure, and the antithrombotic elastic stocking II level (23-32 mmHg) was replaced after 72 h lasting 3 months.
2.4. Follow-Up. The incision healing and skin sensory disturbances were observed 7 days postoperation. Patient followup was done 1, 6, 12, and 24 months postoperation with VCSs, CEAP-C, CIVIQ-14, and varicose recurrence
2.5.Statistical Analysis. The SPSS 24.0, Pearson chi-square test (theoretical number ≥ 5), and the chi-square test for continuity correction (theoretical number < 5 but ≥1) were used for counting data comparison. The measurement data were expressed as mean ± standard deviation and compared with the t-test of independent or paired samples. The Kaplan-Meier method was used to draw the curve of recurrence rate of varicose veins of lower extremities. P < 0:05 was considered statistically signifificant.
3.Results
3.1. Basic Clinical Data. There was no signifificant difffference in age, gender, and other baseline data between the two groups (P > 0:05) (Table 1). No inflfluence of patient obesity on surgical outcomes was observed (Supplement Table 1).
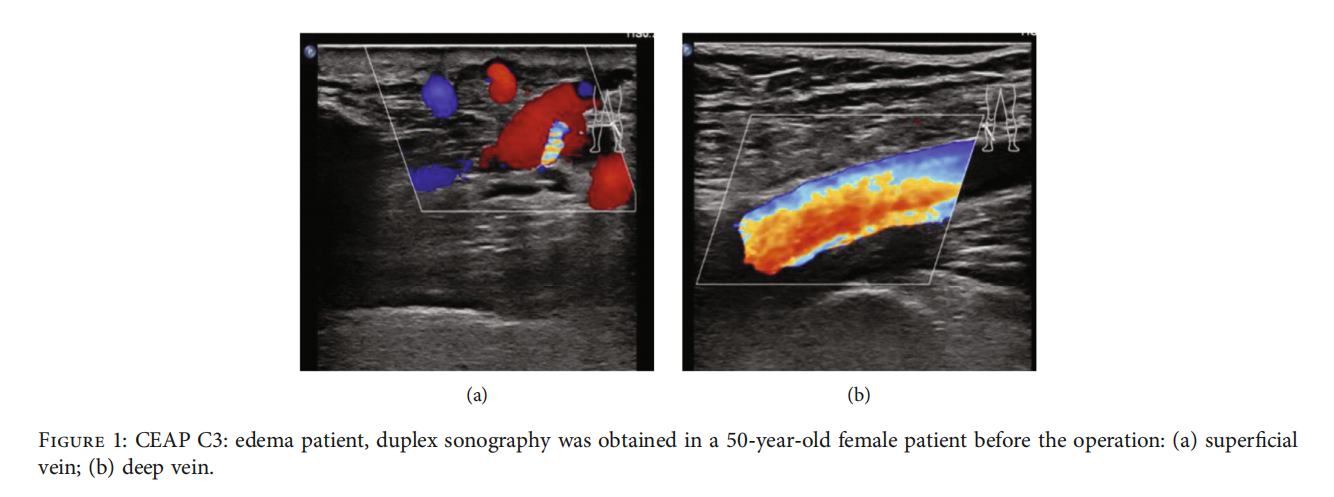
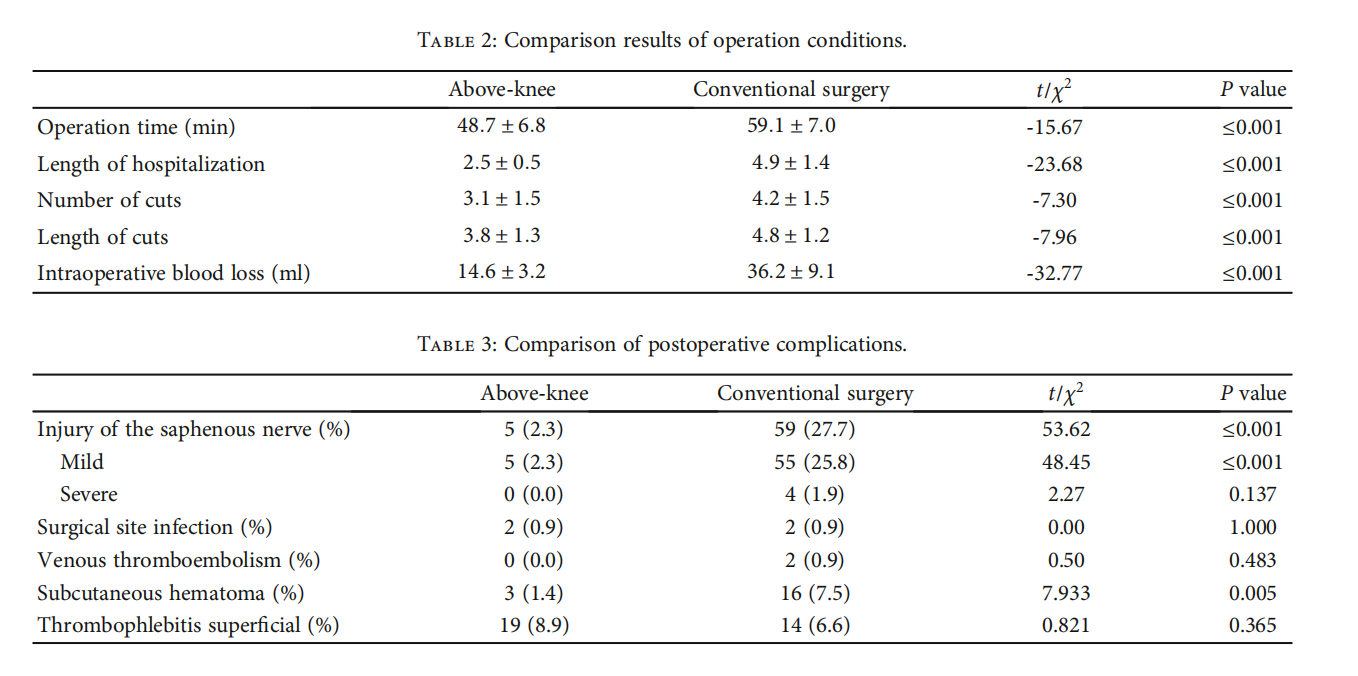
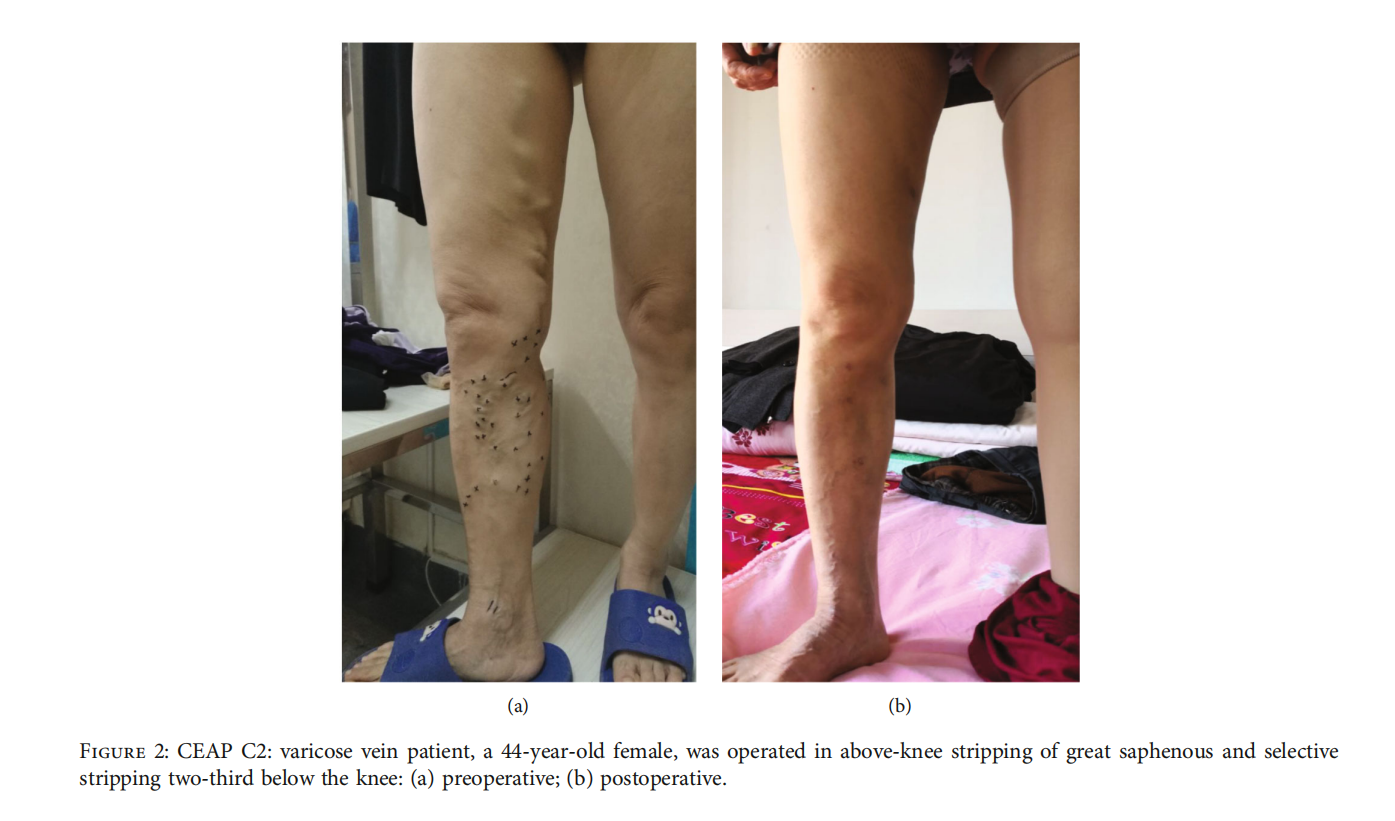
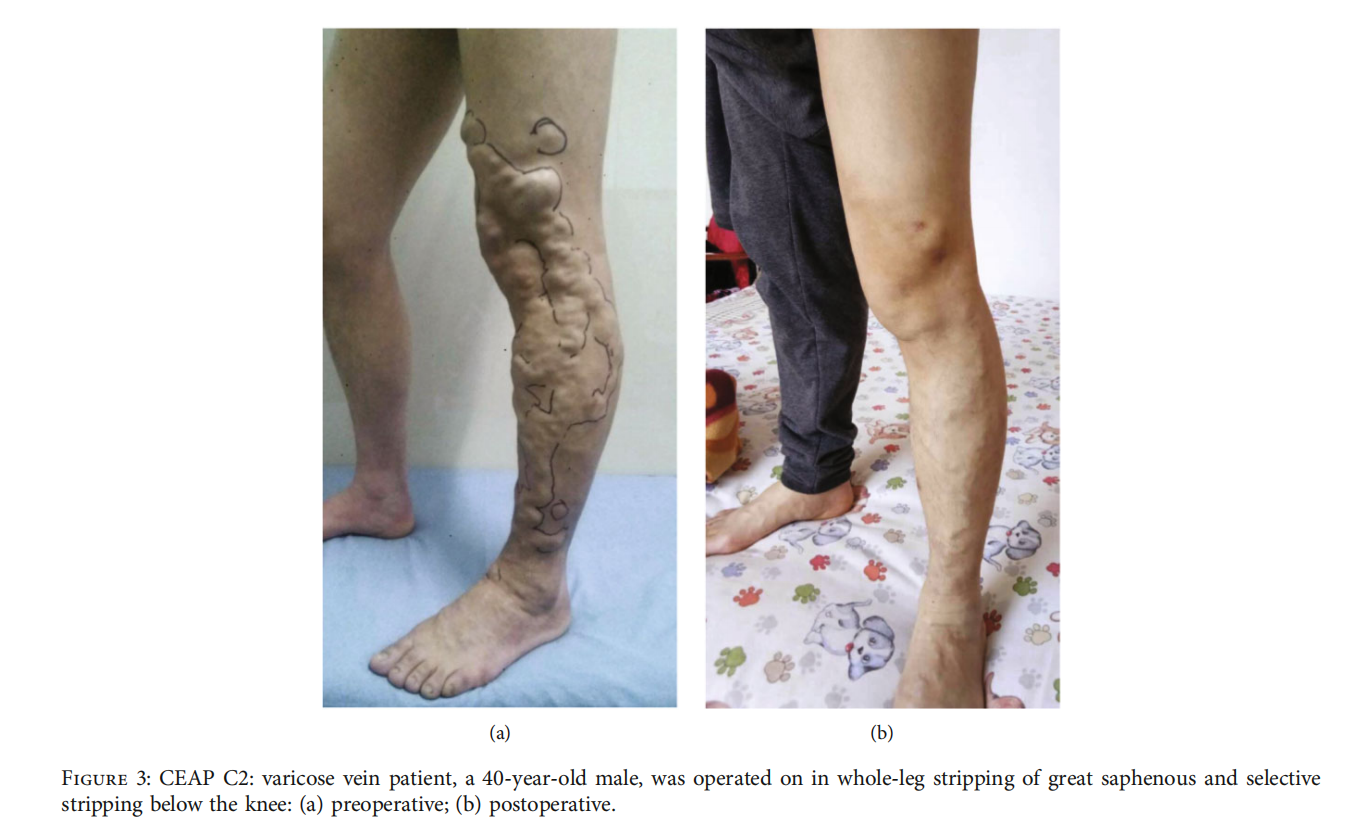
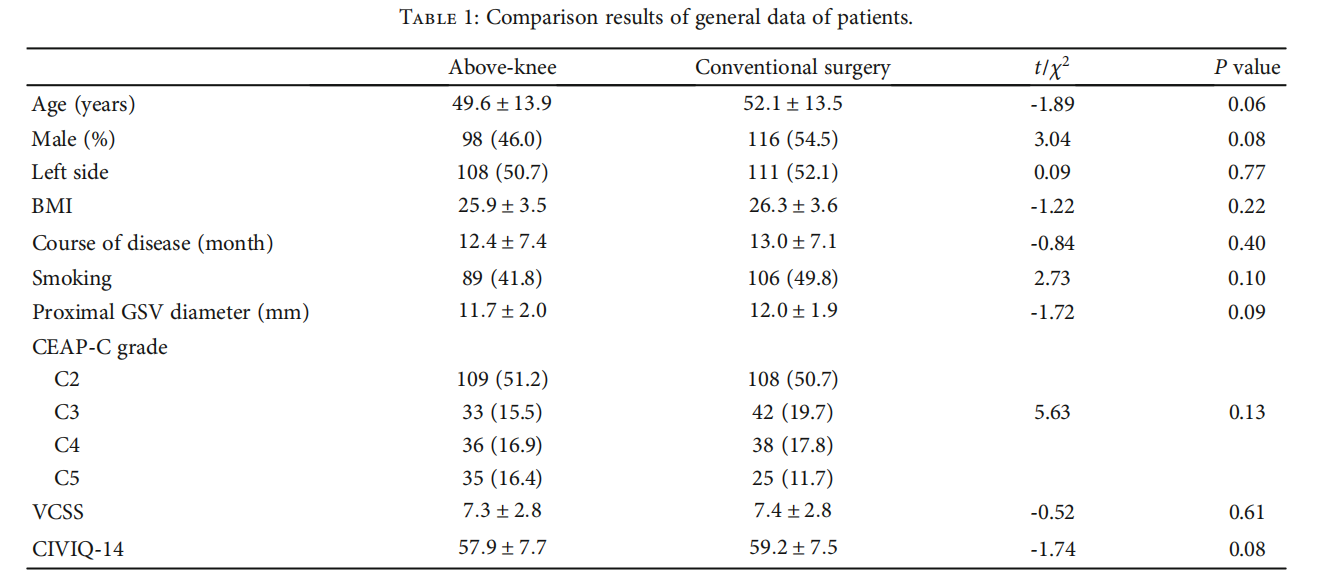 3.2.The local anesthesia rate was 96.5% (411/426), with the intraspinal anesthesia rate at 3.5% (15/426). The technical success rate was 99.1% (211/213) in the aboveknee group and 98.1% (209/213) in the conventional surgery group. The clinical success rate was 97.2% (207/213) in the above-knee group and 97.7% (208/213) in the conventional surgery group. The information before and after surgery is shown in fifigures (Figures 2 and 3). The operation time, hospitalization time, number of incisions, total length of incisions, and intraoperative blood loss in the above-knee group were lower than those in the conventional surgery group (P < 0:05) (Table 2).
3.2.The local anesthesia rate was 96.5% (411/426), with the intraspinal anesthesia rate at 3.5% (15/426). The technical success rate was 99.1% (211/213) in the aboveknee group and 98.1% (209/213) in the conventional surgery group. The clinical success rate was 97.2% (207/213) in the above-knee group and 97.7% (208/213) in the conventional surgery group. The information before and after surgery is shown in fifigures (Figures 2 and 3). The operation time, hospitalization time, number of incisions, total length of incisions, and intraoperative blood loss in the above-knee group were lower than those in the conventional surgery group (P < 0:05) (Table 2).
3.3. The incidence of saphenous nerve injury and subcutaneous hematoma in the above-knee group was lower than that in the conventional surgery group (P < 0:05) (Table 3).
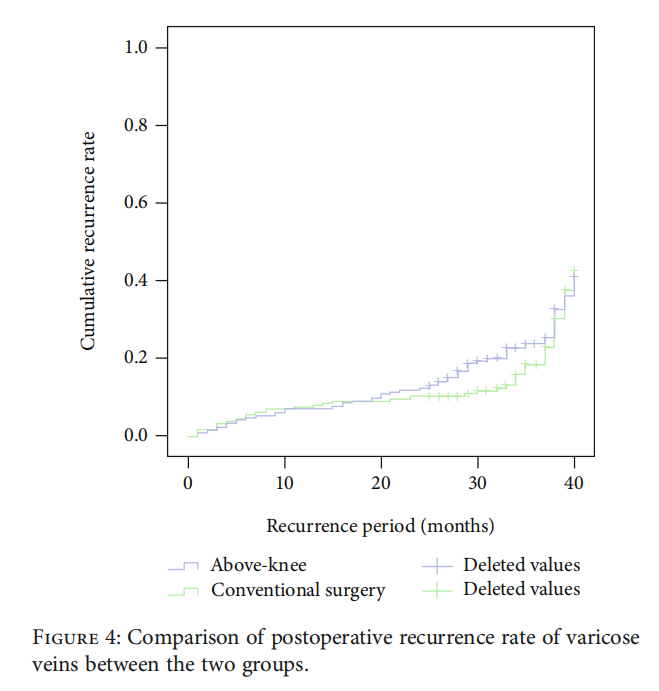
3.4. Recurrence of Postoperative Varicose Vein. The effffective follow-up rate of the above-knee group was 94.4% (201/213), the average follow-up time was (31:5±6:2) months, and the effffective follow-up rate of the conventional surgery group was 93.0% (198/213) with the average follow-up time being (31:6±6:5) months. No difffference was observed in the follow-up time between the two groups (t = −0:145, P = 0:885). The 12 months follow-up showed that the recurrence rates in the above-knee group and the conventional surgery group were 7.0% (15/213) and 7.5% (16/213) (χ2 = 0:035, P = 0:852), respectively. 24 months follow-up showed 12.2% (26/213) and 10.3% (22/213) (χ2 = 0:035, P = 0:852) recurrence rates in the above-knee group and the conventional surgery group, respectively (Figure 4).
3.5.Follow-Up. After 24 months of follow-up, 71.8% (153/213) of patients in the above-knee group and 73.2% (156/213) of patients in the conventional surgery group showed a decrease of CEAP‐C ≥ grade 2. The VCSS score in the above-knee group before operation (7:3±2:8) was higher than one-month postoperation (4:0±1:9) (t = 14:297, P ≤ 0:001). The conventional surgery group before operation (7:5±2:8) was higher than that one month after operation (4:3±1:7) (t = 13:494, P ≤ 0:001). In the above-knee group, the CIVIQ-14 was signifificantly lower before operation (57:9±7:7) than postoperation (84:1±6:9) (t = −35:478,P ≤ 0:001). In the conventional surgery group, the CIVIQ-14 before operation (59:2±7:5) was also signifificantly lower (t = −37:144, P ≤ 0:001) than postoperation (84:9±6:8). There was no signifificant difffference in VCSS or CIVIQ-14 between the two groups before or after operations (P > 0:05) (Table 4).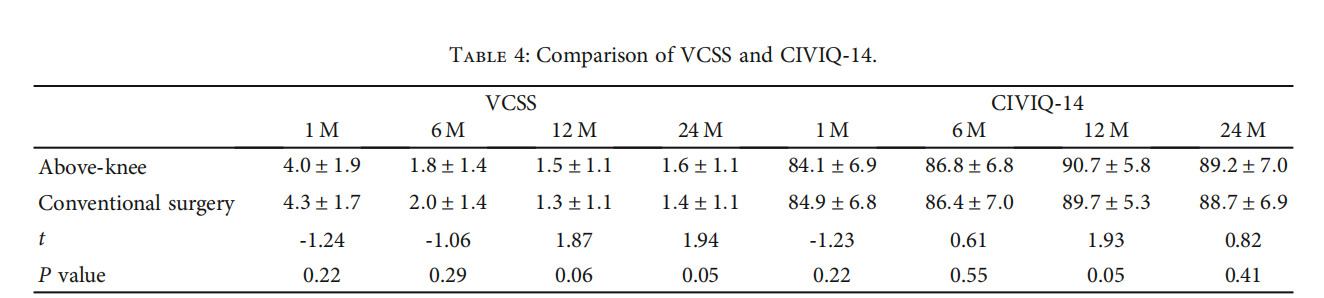
4. Discussion
GSV exfoliation is a standard treatment for varicose veins of the lower extremities [16] with a history of more than 100 years. Although traditional operation effffects are precise, it has been gradually replaced by intracavitary minimally invasive surgery due to the signifificant trauma, slow recovery, and many complications caused by GSV exfoliation [17]. However, the limitation of minimally invasive surgery lies in the high long-term recurrence rate, effffect uncertainties, high price, and the lack of medical insurance access in most regions. This study is aimed at making a clinical comparison between the improved method. The traditional method is not only to ensure the clinical effffect of the traditional method but also to reduce the surgical trauma, the recovery time, and the incidence of complications.
There was no signifificant difffference in baseline characteristics between the two groups. It was reported that a >6 mm GSV proximal diameter was an independent risk factor for endovenous laser recurrence and foam sclerotherapy after varicose veins of lower extremities. However, the recurrence incidence and venous thrombosis increased after radiofrequency ablation when the GSV proximal diameter was>8 mm [18]. Therefore, the defifinition of GSV proximal diameter was set as >8 mm in this study [19, 20]. The anesthetic swelling liquid can play a role in compressing hemostasis and increasing patients’ comfort [21]. Referring to the foreign anesthetic swelling technique report, the rate of operation completion under local anesthesia in this group was 96.5% [22]. In addition, minor trauma and quick recovery were the characteristics of the treatment of residual varicose veins using dot peeling and foam hardening technology instead of traditional open excision [22].
The results show that the modifified above-knee group can reduce the amount of bleeding during the operation and shorten the operation time. The analysis was related to the enormous scope of GSV in the whole limb group and a large amount of bleeding after exfoliation. Local anesthesia was associated with long-term compression while the thigh tissue was loose. The above-knee exfoliation could effffectively compress the GSV hidden room to reduce the amount of bleeding, reduce repeated local anesthesia operation, and save the operation time.
The loss rate during the visit was 6.3% in this present study. The main reason may be associated with patients’ good postoperative recovery without special treatment. The recurrence of varicose veins in the lower extremities was generally divided into three types. Based on the view that the residual and new varicose veins mainly were technical problems or the development of CVD [22], this study takes the recurrence of the true varicose veins that occurred one month after the regional operation as the recurrence standard. The frequency of recurrent varicose veins after surgery varies widely and has been reported to be between 7 and 80% [23, 24]. The overall frequency in our study was 7.0% in the above-knee group and 7.5% in the conventional group.
There was no signifificant difffference in recurrent varicose veins between the two groups at 12 and 24 months after the operation.
The saphenous nerve injury is one of the common complications after varicose vein operation [25]. The pain and numbness caused is a common clinical problem. In this study, the incidence of saphenous nerve injury in the above-knee group was 2.3%, which was signifificantly lower than that in the conventional surgery group (27.7%). Both groups were lower than the relevant literature reports suggesting that distal stripping with vein invagination gives the best saphenous nerve-sparing [25]. It is considered that the removal of GSV from the knee can avoid the saphenous nerve, which is close to the main saphenous vein of the leg, while the removal of the whole limb group can not prevent the excessive tissue damage caused by the traditional exfoliation.
VCSS and CIVIQ questionnaires were authoritative methods for clinical evaluation of the inflfluence of CVD on life quality [26]. This study found that the VCSS and CIVIQ-14 in both groups signifificantly improved after operations. There was no signifificant difffference between the two groups in the score or questionnaire results during the follow-up period, consistent with the relevant literature report [6]. It has also been found that even if the diseased vessels of varicosity of the lower extremity were reopened, the therapeutic effffect and quality of life score would not be affffected [27].
The current single-center retrospective study has all the known limitations of this type of study. Firstly, this is a retrospective observational study with the results not controlled by the investigator during the data accumulation process. Additionally, the completeness and authenticity of the data were diffiffifficult to guarantee, and it was diffiffifficult for patients, surgeons, and follow-up assessors to be blinded to the type of treatment, and the results will inevitably be biased. Lastly, a more extended follow-up period is required to validate these initial results and the safety and durability of this technique.
Collectively, the modifified above-knee technique can ensure clinical outcomes, reduce intraoperative blood loss and the incidence of complications, and shorten the operative time. The modifified above-knee technique is worthy of clinical application.
References
[1] A. Mansilha and J. Sousa, “Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy,” International Journal of Molecular Sciences, vol. 19, no. 6, p. 1669, 2018.
[2] H. Helfer, V. Siguret, and I. Mahe, “Tinzaparin sodium pharmacokinetics in patients with chronic kidney disease: practical implications,” American Journal of Cardiovascular Drugs, vol. 20, no. 3, pp. 223–228, 2020.
[3] A. Rai, M. Porsalman, A. Khatony, and M. Sobhiyeh, “Comparison of foam sclerotherapy versus radiofrequency ablation in the treatment of primary varicose veins due to incompetent great saphenous vein: randomized clinical trial,” Journal of Vascular Nursing, vol. 37, no. 4, pp. 226–231, 2019.
[4] S. Vahaaho, K. Halmesmaki, A. Alback, E. Saarinen, and M. Venermo, “Five-year follow-up of a randomized clinical trial comparing open surgery, foam sclerotherapy and endovenous laser ablation for great saphenous varicose veins,” The British Journal of Surgery, vol. 105, no. 6, pp. 686–691, 2018.
[5] E. Tassie, G. Scotland, J. Brittenden et al., “Cost-effffectiveness of ultrasound-guided foam sclerotherapy, endovenous laser ablation or surgery as treatment for primary varicose veins from the randomized CLASS trial,” The British Journal of Surgery, vol. 101, no. 12, pp. 1532–1540, 2014.
[6] N. Morrison, K. Gibson, M. Vasquez, R. Weiss, and A. Jones,“Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins,” Journal of Vascular Surgery. Venous and Lymphatic Disorders, vol. 8, no. 6, pp. 978–989, 2020.
[7] S. A. S. Hamann, “Response to commentary on “Five-year results of great saphenous vein treatment: a meta-analysis”,” European Journal of Vascular and Endovascular Surgery, vol. 55, no. 5, pp. 742-743, 2018.
[8] N. K. Itoga, K. A. Rothenberg, C. Deslarzes-Dubuis, E. L.George, V. Chandra, and E. J. Harris, “Incidence and risk factors for deep vein thrombosis after radiofrequency and laser ablation of the lower extremity veins,” Annals of Vascular Surgery, vol. 62, no. 45-50, pp. 45–50.e2, 2020.
[9] M. Lawaetz, J. Serup, B. Lawaetz et al., “Comparison of endovenous ablation techniques, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Extended 5-year follow-up of a RCT,” International Angiology, vol. 36, no. 3, pp. 281–288, 2017.
[10] L. Rasmussen, M. Lawaetz, J. Serup et al.,“Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy, and surgical stripping for great saphenous varicose veins with 3-year follow-up,” Journal of Vascular Surgery. Venous and Lymphatic Disorders, vol. 1, no. 4, pp. 349–356, 2013.
[11] C. Nesbitt, R. Bedenis, V. Bhattacharya, G. Stansby, and Cochrane Vascular Group, “Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus open surgery for great saphenous vein varices,” Cochrane Database of Systematic Reviews, vol. 7, article CD005624, 2014.
[12] B. Eklöf, R. B. Rutherford, J. J. Bergan et al., “Revision of the CEAP classifification for chronic venous disorders: consensus statement,” Journal of Vascular Surgery, vol. 40, no. 6, pp. 1248–1252, 2004.
[13] F. Lurie, M. Passman, M. Meisner et al., “The 2020 update of the CEAP classifification system and reporting standards,” Journal of Vascular Surgery. Venous and Lymphatic Disorders, vol. 8, no. 3, pp. 342–352, 2020.
[14] R. B. Rutherford, F. T. Padberg Jr., A. J. Comerota, R. L. Kistner, M. H. Meissner, and G. L. Moneta, “Venous severity scoring: an adjunct to venous outcome assessment,” Journal of Vascular Surgery, vol. 31, no. 6, pp. 1307–1312, 2000.
[15] T. Sinozic, K. Bazdaric, D. Sverko, A. Ruzic, and M. Katic, “Validation of the Croatian version of CIVIQ quality of life questionnaire in patients with chronic venous disorders,” Croatian Medical Journal, vol. 58, no. 4, pp. 292–299, 2017.
[16] P. Pittaluga, S. Chastanet, and J. J. Guex, “Great saphenous vein stripping with preservation of sapheno-femoral conflfluence: hemodynamic and clinical results,” Journal of Vascular Surgery, vol. 47, no. 6, pp. 1300–1304, 2008.
[17] K. Rass, N. Frings, P. Glowacki, S. Graber, W. Tilgen, and T. Vogt, “Same site recurrence is more frequent after endovenous laser ablation compared with high ligation and stripping of the great saphenous vein: 5 year results of a randomized clinical trial (RELACS study),” European Journal of Vascular and Endovascular Surgery, vol. 50, no. 5, pp. 648–656, 2015.
[18] N. Sermsathanasawadi, W. Pitaksantayothin, N. Puangpunngam et al., “Incidence, risk factors, progression, and treatment of endovenous heat-induced thrombosis class 2 or greater after endovenous radiofrequency ablation,” Dermatologic Surgery, vol. 45, no. 4, pp. 573–580, 2019.
[19] J. Toniolo, N. Chiang, D. Munteanu, A. Russell, H. Hao, and J. Chuen, “Vein diameter is a predictive factor for recanalization in treatment with ultrasound-guided foam sclerotherapy,” Journal of Vascular Surgery. Venous and Lymphatic Disorders, vol. 6, no. 6, pp. 707–716, 2018.
[20] P. F. Lawrence, A. Chandra, M. Wu et al., “Classifification of proximal endovenous closure levels and treatment algorithm,” Journal of Vascular Surgery, vol. 52, no. 2, pp. 388–393, 2010.
[21] M. L. Wall, C. Dealey, R. S. Davies, and M. H. Simms, “Local versus general anaesthesia for varicose veins surgery: a prospective non-randomized controlled trial,” Phlebology, vol. 24, no. 2, pp. 61–66, 2009.
[22] N. Haruta, “Recent progress of varicose vein treatment especially about endovascular heat ablation, SEPS and foam sclerotherapy,” Annals of Vascular Diseases, vol. 11, no. 1, pp. 66–71,
[23] M. R. Perrin, N. Labropoulos, and L. R. Leon Jr., “Presentation of the patient with recurrent varices after surgery (REVAS),” Journal of Vascular Surgery, vol. 43, no. 2, pp. 327–334, 2006.
[24] M. Brake, C. S. Lim, A. C. Shepherd, J. Shalhoub, and A. H. Davies, “Pathogenesis and etiology of recurrent varicose veins,” Journal of Vascular Surgery, vol. 57, no. 3, pp. 860– 868, 2013.
[25] A. Jaworucka-Kaczorowska, G. Oszkinis, J. Huber, A. WiertelKrawczuk, E. Gabor, and P. Kaczorowski, “Saphenous vein stripping surgical technique and frequency of saphenous nerve injury,” Phlebology, vol. 30, no. 3, pp. 210–216, 2015.
[26] M. A. Vasquez, J. Wang, M. Mahathanaruk, G. Buczkowski, E. Sprehe, and H. H. Dosluoglu,“The utility of the venous clinical severity score in 682 limbs treated by radiofrequency saphenous vein ablation,” Journal of Vascular Surgery, vol. 45, no. 5, pp. 1008–1015.e2, 2007.
[27] L. Rasmussen, M. Lawaetz, L. Bjoern, A. Blemings, and B. Eklof, “Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years,” Journal of Vascular Surgery, vol. 58, no. 2, pp. 421–426, 2013.
Acknowledgments
The study is funded by the Medical Science Research Project of the key research and development program of Hebei Province, China (No: 20377732D), and the Medical Science Research Project of Health and Family Planning Commis sion of Hebei Province, China (No: 1020140360).


