Issue: Volume 62 - Issue 4 - April 2016 ISSN 1943-2720
Index: Ostomy Wound Manage. 2016;62(4):42-52.
Login or Register to download PDF
Abstract
Heels are susceptible to pressure ulcer (PU) development. Some evidence suggests dressings may provide mechanical cushioning, reduce friction with support, and lower localized internal tissue loading, which together may minimize the risk for heel ulcers (HUs). To examine the effect of dressing application on pressure ulcer prevention, 20 computer simulations were performed.Volumetric exposure of soft tissues to effective and shear strains and stresses, with and without a multilayered foam dressing, were assessed, with the extent of tissue exposure considered as measures of the theoretical risk for PUs. The simulations, conducted using the finite element method, provided the mechanical strain and stress magnitudes and distributions in the weight-bearing tissues of the heel, which were visualized and analyzed post-hoc for comparing diabetic to healthy tissue loads with/without prophylactic dressings and at different foot (plantar flexion) postures. The volumetric exposure of the soft tissues of the heel to elevated strains and stresses was considerably reduced by the presence of the dressing, whether diabetic or nondiabetic tissue conditions existed, and for the entire range of the simulated plantar flexion positions. Further, greater plantar flexion, which occurs with elevation of the head of the bed, reduced the volumetric exposure of subcutaneous fat to increased effective strains and stresses, again, particularly when the dressing was on. Specifically, peak (maximum of raw data) effective strains in the soft tissues of the heel decreased by 14.8% and 13.5% with the use of the dressing for healthy persons and persons with diabetes, respectively. Additionally, volumetric exposures of the soft tissues to large effective strains, defined as exposures to >50% strain, decreased substantially, by at least a factor of 2, with the angle of plantar flexion and with respect to a neutral foot posture. Volumetric exposures to midrange (<50%) strains were more mildly affected by the foot posture (ie, <10% difference was noted across plantar flexion angles). The differences in tissue exposures to strains and stresses between the dressing and nondressing models suggest this dressing provides an important biomechanical protective effect, specifically when diabetic tissue conditions exist. In addition, the data suggest alleviating shear by repositioning the heels after elevating the head of the bed may be critical in order to limit the increase in tissue stress and subsequent PU risk. Randomized, controlled clinical studies to examine the efficacy of dressings for the prevention of heel PUs are warranted.
Introduction
Surveys1-4 show heel ulcers (HUs) are the most common type of facility-acquired pressure ulcers (PUs), and the heels are the most common site for deep tissue injury (DTI).Clinical trials have identified PUs as generally being associated with a number of contributing or confounding factors, such as impaired mobility and sensory capacities, compromised perfusion, increased body mass index, and type 2 diabetes, to name a few.5,6 During prolonged supine bed rest, the weight of the foot and ankle is transferred through the calcaneus bone to the support surface, subjecting subcutaneous fat and skin near the heel-support contact site to sustained mechanical loads, as demonstrated theoretically and in computer simulations.7 In lack of mobility or sensitivity or both, the sustained tissue loads may exceed tissue tolerance levels, increasing the risk for HUs.7 In persons with type 2 diabetes, in particular, peripheral sensory neuropathy may prevent patients from detecting the onset and progression of tissue damage, which has been documented in numerous clinical cases.8
Finite element (FE) computational modeling is a powerful tool in PU research (see Sidebar: Finite Element Computational Modeling). FE modeling facilitates quantification of internal tissue strains and stresses in weight-bearing body parts such as the heels and buttocks.6,9-12 This method is further able to isolate the influence of specific intrinsic and extrinsic biomechanical factors on the resulting risk for PUs and DTIs, as demonstrated in various previous computational modeling studies.10-13 Briefly, the 3-dimensional (3D) anatomical model, usually derived from medical imaging such as magnetic resonance imaging (MRI), is divided into hundreds of thousands of small elements in the computer. The material properties and loading configuration is defined for each simulation; the computer then is able to calculate the mechanical reaction of the model for each element with respect to its neighboring elements, ultimately constructing the solution of the biomechanical problem for the entire structure. For example, in a previous study from the authors’ group, Sopher et al12 employed FE computational modeling to investigate how variation of tissue stiffness properties and degree of external rotation of the foot may affect the internal state of fat and skin strains and stresses in the resting heel for supine body postures. The authors demonstrated how lateral inclination of the foot might jeopardize tissue integrity by exerting higher strains and stresses on the soft tissues of the lateral aspect of the heel; however, their study did not include representation of the anatomical site of insertion of the Achilles tendon into the calcaneus bone nor did it examine the effect of diabetic tissue conditions or shearing forces acting on the foot.
Type 2 diabetes has been experimentally associated with stiffening of collagen-rich soft tissues, an effect demonstrated in both animal models and testing of human tissue specimens, as reviewed in detail in the modeling study of Gefen,8 which was focused on the influence of such connective tissue stiffening on the function of the foot. Mechanical testing8,14-16 consistently demonstrated stiffening of fat and skin; however, Connizzo et al17 reported reduced stiffness of the tendon tissue at the insertion site of the Achilles tendon into the calcaneus of mice. Because this insertion site is compositionally and structurally different from the midsubstance of the tendon, diabetes-related alterations at the insertion region may be expressed differently than at other parts of the tendon.
Given that PUs (and HUs in particular) are a major burden for health care facilities and that apart from their devastating impact on the quality of life of sufferers these wounds are known to be difficult and costly to treat, all of which has been documented extensively in patient safety, quality-of-life and health-economy human studies,18-20 great efforts are focused on prevention. Although complete offloading of the heels still is considered the most effective method for preventing HUs,7 it is not always feasible in the long-term, especially when considering patient movement. An illustrative example is a patient who can be immobile for hours but from time to time suffers seizures that cause his legs to move and change position.
A prevention concept that is attracting the attention of several research groups around the world is the prophylactic use of dressings in order to protect the heels from HUs.7,21,22 In the past few years, the efficacy of such dressings in preventing HUs has been confirmed by more than 20 different clinical studies summarized in a recent systematic review22 that included randomized controlled trials (RCTs), cohort, and case series studies. In addition, positive reports have been published of contact pressure and shear measurements in experimental (laboratory) models.23-26 Recently, the development was reported of a 3D, MRI-based FE computational model for investigating the internal mechanical strains and stresses in the soft tissues of the supported heel and how these are influenced by the use of a prophylactic dressing.27Although this research did not consider diabetic tissue conditions, clinicians often consider diabetes as a risk factor for HUs6 more because of the patient’s impaired sensation and perfusion and less because the biomechanics of the soft tissues are altered. Nevertheless, theory and computer modeling show abnormal biomechanical properties of diabetic connective soft tissues, where the tissues cannot adequately deform to dissipate the body loads, play a role in the overall risk of injury.8
In addition to prophylactic dressing use, another consideration is the posture of the ankle relative to bed elevation. A case-control study by Singer et al28 suggested the relaxed posture of the ankle in supine lying is external rotation with relative plantar flexion, but elevation of the head of the bed may increase the angle of plantar flexion in the relaxed ankle joint. Furthermore, in a review of relevant case series studies, guidelines, observational studies, and expert opinions, Fowler et al29 concluded elevating the head of the bed may induce additional shearing forces on the weight-bearing soft tissues of the heels, which should be examined in combination with diabetic tissue conditions given the fragility of the diabetic heel.
Mepilex® Border Heel (Mölnlycke Health Care, Gothenburg, Sweden) is a 5-layer dressing consisting of 1) a backing film which faces the support, 2) an airlaid layer, 3) a nonwoven layer, 4) a polyurethane foam layer, and 5) a Safetac® layer that adheres to the skin.27 This specific dressing is indicated for both prevention and treatment of pressure ulcers and has already been used in randomized clinical trials5,24 where it was shown to be effective as a prophylactic intervention.
The purpose of this study is to expand the authors’ previously published work27 that demonstrated the biomechanical efficacy of a prophylactic dressing in protecting the heels in order to account for diabetic tissue conditions. For this purpose, sets of computational model variants of the heel in supine lying were developed to represent patients with diabetes with their heels resting in a range of plantar flexion positions, as well as corresponding simulations of healthy tissue conditions.
Methods
Geometry. A set of 20 FE computational model variants was developed, representing diabetic tissue conditions and comparable healthy tissue cases at different foot postures, as specified in Table 1. Each model variant captured the anatomy of the posterior aspect of the heel, including the calcaneus bone, distal Achilles tendon, fat, and skin (see Figure 1a). Flat elastic foam was modeled as the support surface (see Figure 1a). Ten (10) of the model variants did not include the multilayered dressing and were used as reference cases, which then could be compared to cases where the foam dressing was incorporated (see Table 1). Variants 1–4 were set to a neutral foot position (0˚ plantar flexion) and variants 5–8, 9–12, and 13–20 were set to 10˚, 20˚, and 30˚ of plantar flexion, respectively (see Table 1, Figure 1). For each position of the foot tested, either diabetic or healthy soft tissues properties were assigned.
The anatomical structures of the posterior heel from the MRI dataset, as well as geometrical modeling of the dressing, were segmented as described in the authors’ previous work.27 Briefly, 56 T1-weighted axial MRI slices from the suspended left heel of a healthy man were used to develop an anatomically realistic, 3D, undeformed model geometry, using the ScanIP® module of the Simpleware® software package30,31(Simpleware, Exeter, United Kingdom). For clinical relevance, the study dressing was modeled (the specific considerations regarding the geometrical and material modeling of the dressing have been published previously27). The innermost and outermost layers of the dressing were modeled as contact conditions and used according to the manufacturer’s specifications to describe the airlaid (layer 2), nonwoven (layer 3), and polyurethane foam (layer 4) as physical layers in the modeling. The flat foam support (10-mm thickness) was added to all the heel model variants at the preprocessing stage in the PreView module of the FEBio software package.32
Mechanical properties of the tissues and dressing. Constitutive laws and mechanical properties of all the tissues included in the heel model were adopted from the literature (see Table 2). Specifically, the healthy Achilles tendon and calcaneus bone were assumed to be linear-elastic isotropic materials (ie, having a constant stiffness that does not depend on the extent of deformation nor does it depend on the direction of the deformation) with instantaneous elastic moduli of 205 kPa and 7 GPa, and Poisson’s ratios (indicating a material’s deformation at a direction perpendicular to the loading direction) of 0.49 and 0.3, respectively33,34 (see Table 2). Skin and fat tissues were assumed to be nearly incompressible (Poisson’s ratio of 0.495), nonlinear isotropic materials. The large deformation mechanical behavior of skin and fat was described by an uncoupled Neo-Hookean material model35 with instantaneous shear moduli33,36 (see Table 2). Based on the literature, the instantaneous shear moduli of diabetic skin and fat tissues were assumed to be 40% stiffer than the corresponding healthy tissue14; likewise, the diabetic Achilles tendon was assigned a 40% softer instantaneous elastic modulus17 (see Table 2). The flat foam support was assumed to be isotropic linear elastic with a Poisson’s ratio of 0.3 and an elastic modulus of 45 kPa, which are within the range of hospital mattress properties.37 Layers 2, 3, and 4 were assigned instantaneous elastic moduli of 15.3, 75, and 12 kPa, respectively, according to the measurements described previously27(see Table 2). The Poisson’s ratios of all dressing materials38 were set as 0.258 (see Table 2).
Body loads applied to the model, shear, and friction conditions. The loads applied to the model were chosen to simulate the descent of the calcaneus bone against a flat foam support during supine weight-bearing. For calibration, tissue deformations were measured from a weight-bearing MRI of the heel of the same subject to find the weight force the heel applied on the support. Because the total reaction force should be equal to the weight of the foot/ankle in the supine position, this datum was used to produce the same heel-support reaction forces in all the model variants. The resulting compressive (downward) displacements after imposing the same foot/ankle weight value in all the model variants were in the 4.7-mm to 5.2-mm range. Additionally, in order to represent shearing forces acting on the foot when the head of the bed is elevated, and by doing so to evaluate the importance of subsequent heel repositioning in patients with and without diabetes, model variants 17–20 were used. In these specific variants, a combination of compression and shear loading was employed, where shear displacements were taken as equal to the compressive ones27 (see Table 1). The bottom surface of the elastic foam support was fixed for all motions. Tied (fixed) interfaces were defined between all tissue components as well as between the skin and the polyurethane foam layer of the dressing (layer 4) to account for the adherence properties of the innermost Safetac® layer of the dressing that interfaces the skin (layer 5). Frictional contact between the support and either the skin of the bare heel or the outer surface of the dressing was defined, with the coefficients of friction set as 0.43 and 0.35 for the skin-support and dressing-support contacts, respectively.27,38,39
Creating the computational simulations. The tissues and dressing were meshed using the ScanIP®module of Simpleware®,30 with mesh refinements that were applied in the skin and dressing materials near the contact area with the support (see Figure 1a). Meshing the support was performed in the Preview module of FEBio.32 All of the above FE simulations were set up using the PreView module of FEBio (Version 1.14), analyzed using the Pardiso linear solver of FEBio (http://mrl.sci.utah.edu/software/febio) (Version 1.7.1) and post-processed using the PostView tool of FEBio (Version 1.4).32
Biomechanical outcome measures. The volumetric exposures of the soft tissues to mechanical strains were systematically compared using the effective Green-Lagrange strain as the strain measure.35 The effective Green-Lagrange strain is an adequate measure of large tissue deformations and the consequent risk for HUs because it is simultaneously considering tissue deformations in all loading modes (ie, compression, tension and shear) using a single weighed (scalar) measure. The strain data were pooled together from tendon, fat, and skin tissues for all elements in a consistent volume of interest in the meshes, which was defined by the circumference of the calcaneus and its projection at the retrocalcaneal region, as illustrated in Figure 1b. Additionally, peak effective and maximal shear stresses (shear stresses arise from shape distortion of the tissues due to forces parallel to the cross-sections of the tissues) in fat and skin tissues were compared separately as a second measure of the risk for HUs. For clarification, effective strain and stress measures consider compression, tension, and shear loads altogether, as opposed to just the aforementioned (maximal) shear measures, which do not consider tension and compression loads. The stress data were pooled from all the elements of skin or separately from those of fat. Converged time steps for data collection were chosen so the resulting reaction forces acting back from the support were within <3% difference from the predefined target reaction force (as explained previously). Hence, the biomechanical efficacy of the dressing was evaluated in diabetic and healthy tissue conditions based on the criteria of reduction in volumetric exposures of the soft tissues to critical effective and shear strains and stresses. The verification of the reference model variant based on the calculated interface pressures under the heel and corresponding compressive strains in the support, which have been compared to published experimental data, is described in a previous paper by the authors that fully validates the present modeling framework.27
Data collection and analyses. Excel software (Microsoft Co, Spokane, WA) was used for the post-hoc analyses of the finite element simulations and for plotting and quantitatively comparing the results of all simulations. It should be mentioned that means and standard deviations are not provided because no variability in the modeling outcomes exists when the parameters of the models are set at a certain way (see Sidebar). Accordingly, no variance around the mean and no average values are reported — just a single value of each parameter that is calculated from a predefined set of simulation parameters (eg, peak tissue strain is calculated based on a simulated set of specific tissue mechanical properties and whether a dressing is present or not). The standard deviations are zero; each time the same simulation is run, the same outcome is obtained.
Results
Effective strain distributions in the soft tissues of the heel in a neutral position and in 10˚, 20˚, and 30˚ plantar flexion, without and with the protection of the dressing, are shown in Figure 2 and Figure 3, respectively. Consistent with the authors’ previous work,27 peak effective strains were found at the bone-fat interface in all the model variants and these were shifted distally with an increase in plantar flexion (see Figures 2, 3). Specifically, peak (maximum of raw data) effective strains in the soft tissues of the heel decreased in presence of the dressing by 14.8% and 13.5% for the healthy persons and persons with diabetes, respectively. Additionally, volumetric exposures of the soft tissues to large effective strains, defined as exposures to >50% strain, decreased by at least a factor of 2 with the angle of plantar flexion and with respect to the neutral position of the foot. Volumetric exposures to midrange (<50%) strains were more mildly affected by the foot posture (ie, <10% was noted difference across plantar flexion angles) (see Figure 4).
Substantial alleviation of internal peak effective tissue stresses when the dressing was on, with respect to corresponding conditions when it was off, was evident in all foot positions for both the diabetic and healthy tissue conditions (see Figure 5); the most pronounced effect occurred in skin tissue that was subjected to less intense deformation exposures (see Figure 5 – dressing on versus dressing off). Specifically, peak effective stresses in the soft tissues of the heel decreased by 25.5% and 22.2% in the presence of the dressing for healthy and diabetic cases, respectively. Overall, diabetic tissue conditions yielded greater effective and shear stresses in skin and fat tissues and lower stresses in the Achilles tendon with respect to healthy corresponding tissues (see Figure 5).
Effective stress distributions on the exterior skin surface in a neutral foot position after simulated elevation of the head of the bed and following subsequent repositioning of the heel are shown in Figure 6. The substantial increases in effective and shear stresses on the skin with the elevation of the head of the bed are considerably restrained by the use of the dressing; exposure to skin stresses is reduced by a factor of 2 to 3, and exposure to fat stresses is reduced by a factor of ~1.5 with application of the dressing, in either nondiabetic or diabetic tissue conditions (see Figures 6, 7). Furthermore, shear alleviation through repositioning of the heel was able to reduce the effective and shear stresses developing in skin tissues in diabetic and healthy cases between 20% and 40% across the different cases (see Figure 7), which is substantial. This occurred either with or without the presence of the dressing (see Figures 6, 7a,b), which points to the importance of offloading (elevating) the heels whenever shear in tissues is suspected (eg, immediately after the head of the bed is elevated).
In general, volumetric exposure of fat tissue to effective stresses decreased with the angle of plantar flexion with respect to a neutral foot position and with elevation of the head of the bed. Nevertheless, alleviating shear by repositioning the heels was able to further reduce the volumetric exposures of fat tissues to effective stresses exceeding critical levels12 with regard to a state where no offloading has been performed (see Figure 7c). This again points to the importance of repositioning the heels — elevating and then gently resting them on the mattress again whenever internal tissue shear is suspected, such as in cases where the head of the bed has been lifted or where a patient appeared to have slipped down in the bed. Doing so is still important even if prophylactic dressings are being used (see Figure 7). 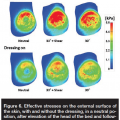
Discussion
This study employed 20 MRI-based FE model variants of the heel in a supine posture for a range of plantar flexion positions in order to investigate the biomechanical efficacy of a prophylactic heel dressing in preventing HUs if diabetic tissue conditions exist. In accordance with the authors’ previous work,27 the heel dressing was found to consistently and considerably reduce the volumetric exposure of the soft tissues of the heel to elevated strains and stresses with respect to a bare heel condition (see Figures 2–7), whether diabetic or nondiabetic tissue conditions existed, and for the entire range of the simulated plantar flexion positions. Further, greater plantar flexion reduced the volumetric exposure of subcutaneous fat to increased effective strains and stresses. This finding can be explained by the thicker subcutaneous fat layer facing the support surface and the growing distance of the heel-support contact site from the insertion area of the (stiffer) Achilles tendon as the degree of plantar flexion increases.12,31 However, skin tissues seem to be conversely affected. As the angle of plantar flexion increases and subcutaneous fat deformations decrease, greater effective and shear stresses occur in skin tissues (see Figure 7a,b). Nevertheless, the dressing was able to considerably limit that increase in effective and shear stresses in the skin with respect to a bare heel condition to the point where the use of the dressing in plantar flexion of 30˚ resulted in lower skin stresses than for a neutral position without the dressing. This result was consistently observed for both diabetic and nondiabetic tissue conditions, which may suggest the use of bed footrests to maintain the foot in minimal plantar flexion is redundant if the dressing is placed for protection. However, this is subject to additional clinical studies.
Elevating the head of the bed, some spontaneous movements of the patient, or repositioning that has not been carefully performed all can result in an increased plantar flexion angle of the foot and the addition of shear forces acting on the supported posterior heel. As expected, such additional shear forces were shown in this study to substantially exacerbate the states of effective and shear stresses in skin tissues. Based on this study, temporarily lifting (offloading) and then repositioning the heels on the support surface immediately after elevating the head of the bed or, in general, after repositioning, is crucial to tempering this increase in skin stresses. Furthermore, patients who are positioned in bed with their heads elevated (eg, to improve respiration) tend to slide down on the bed over time due to gravity, which then causes the development of recurrent shear loads in the weight-bearing soft tissues of the heels (and in the buttocks as well).40 Based on the present findings, the use of the dressing should theoretically minimize skin and subcutaneous fat stresses, even if shear loads redevelop over time (see Figure 7).
Questions have been raised regarding the modes of action and the sustainability of the protective effect of the dressing, and some bench tests were performed in this regard.38 In the current simulations, the cushioning effect of the dressing is formally calculated for a short period (in the order of minutes) following a positioning/repositioning action; however, results can still be generalized and extrapolated to longer times (ie, in the order of several hours) of immobility, within which transient viscoelastic phenomena typically plateau in tissues. This theoretical extrapolation can be made because the instantaneous dressing and tissue stiffness properties were considered, thereby describing the worst-case scenario in terms of exposure to internal tissue strains and stresses.6,27,41 Also, the authors’ previously published laboratory findings27 show negligible viscoelasticity of the dressing materials, so the cushioning effect of the dressing should persist, which further supports the above point.
Based on animal model studies as well as mechanical tests of human tissue specimens, which are reviewed in Gefen,6,8 the altered mechanical properties of the skin, subdermal fat, and Achilles tendon in diabetes appear to play an important role in the etiology of HUs (and PUs in general). Although increased soft tissue stiffness (as in skin and fat) increases the risk of elevated tissue stresses, abnormal softening of tissues (such as at the insertion of the Achilles) exposes the affected and nearby tissues to an increased risk of deformation-inflicted injury. Reducing localized tissue deformation is particularly important in diabetes, because low deformation levels are needed to protect the vasculature in a situation where perfusion often already is compromised.42,43
Limitations
Computational FE modeling is a powerful tool in PU research but it is not without limitations that originate from the assumptions and omissions made. First, the mechanical properties of the tissues are taken from animal studies. Furthermore, skin and fat tissues are considered hyperelastic. The Achilles tendon and the dressing materials are considered linear elastic; hence, any transient biomechanical viscoelastic phenomena are omitted in the modeling.
Additionally, the reference model variant is based on anatomical data of a single young healthy subject, which does not necessarily represent all possible heel structures in terms of dimensions, sites, neutral external rotation, and the like. Moreover, the mechanical properties of healthy and diabetic soft tissues are considered homogeneous, although the alterations in tissue stiffness properties associated with diabetes are hardly homogeneous. Local stiffening of connective tissues is occurring as mechanical lesions, which exert greater localized tissue stresses as well as greater strains in the softer, adjacent regions of the affected tissues, and which then act to increase the risk of local tissue damage.
Conclusion
In this study, the differences between models of a bare heel and models covered with the modeled dressing were considerably different in soft tissue exposures to internal tissue loads, where loads in presence of the dressing were lower. This suggests the dressings provide a cushioning effect to the soft tissues of the heel, as well as that they temper deformations from the tissues by deforming internally themselves in shear mode, hence consistently lowering exposure to hazardous levels of strains and stresses in the simulations in a variety of foot postures and tissue conditions including the case of diabetic tissue conditions. Increasing plantar flexion angles of the resting foot was found to result in safer mechanical states (lower tissue strain and stress exposures) for subcutaneous fat, but more plantar flexion also could worsen the loading state in skin if offloading the heels is not performed in a timely manner. However, the use of the dressing protected skin tissues by lowering the effective and shear stresses in skin for these plantar flexed positions compared to a bare heel state. It is still recommended to offload (ie, temporarily elevate) the heels immediately after elevation of the head of the bed, even if the dressings are used to protect the heels. This is in order to minimize shear loads in the soft tissues of the heels (and buttocks), which are formed as a consequence of elevating the head of the bed or other repositioning maneuvers, as the body tends to slide down in the bed, due to gravity.40 The results of this study suggest repositioning the heels immediately after elevating the head of the bed to mitigate the effects of gravity and then repeating this repositioning from time to time afterwards may be an important measure to reduce the risk of PU development. Randomized controlled clinical studies to examine the efficacy of dressings for the prevention of heel PUs are warranted.
Disclosure
Dr. Gefen is Chair of the Scientific Advisory Board of Mölnlycke Health Care, Gothenburg, Sweden and is funded by Mölnlycke Health Care for investigating the effects of dressing materials and designs on soft tissues during weight-bearing.
Affiliations
Ms. Levy is a doctoral student; and Dr. Gefen is a Professor in Biomedical Engineering, Department of Biomedical Engineering, Faculty of Engineering, Tel Aviv University, Tel Aviv, Israel.
Correspondence
Please address correspondence to: Amit Gefen, PhD, Tel Aviv University, Biomedical Engineering, Ramat Aviv, Tel Aviv 6997801 Israel; email: 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。.


