1. Introduction
Nicotinamide adenine dinucleotide (NAD+ ), one of the most indispensable biological molecules, has been intensively studied in recent decades because of its importance in cellular metabolism. NAD+ participates in redox reactions by transferring electrons between NAD+ (the oxidized form of NAD) and NADH (the reduced form of NAD). It acts as a cofactor for enzyme reactions involving poly(ADP-ribose) polymerases (PARPs), sirtuins, and NAD glycohydrolases (CD38 and CD157) [1,2]. Due to its biological importance, NAD+ biosynthesis and its related metabolic pathways are highly conserved between yeast and vertebrates [3]. NAD+ biosynthesis is maintained through three pathways: de novo synthesis, which converts tryptophan into quinolinic acid (QA); NAD+ , nicotinamide (NAM)/nicotinic acid (NA) salvage; and nicotinamide riboside (NR) salvage [4]. Despite its biological significance, the precise roles of NAD+ and its downstream targets remain unclear. Studies over the past two decades have highlighted its roles and actions in the aging process [5,6]. They have demonstrated an age-dependent decline in NAD+ levels and their association with the hallmarks of aging and age-related diseases.
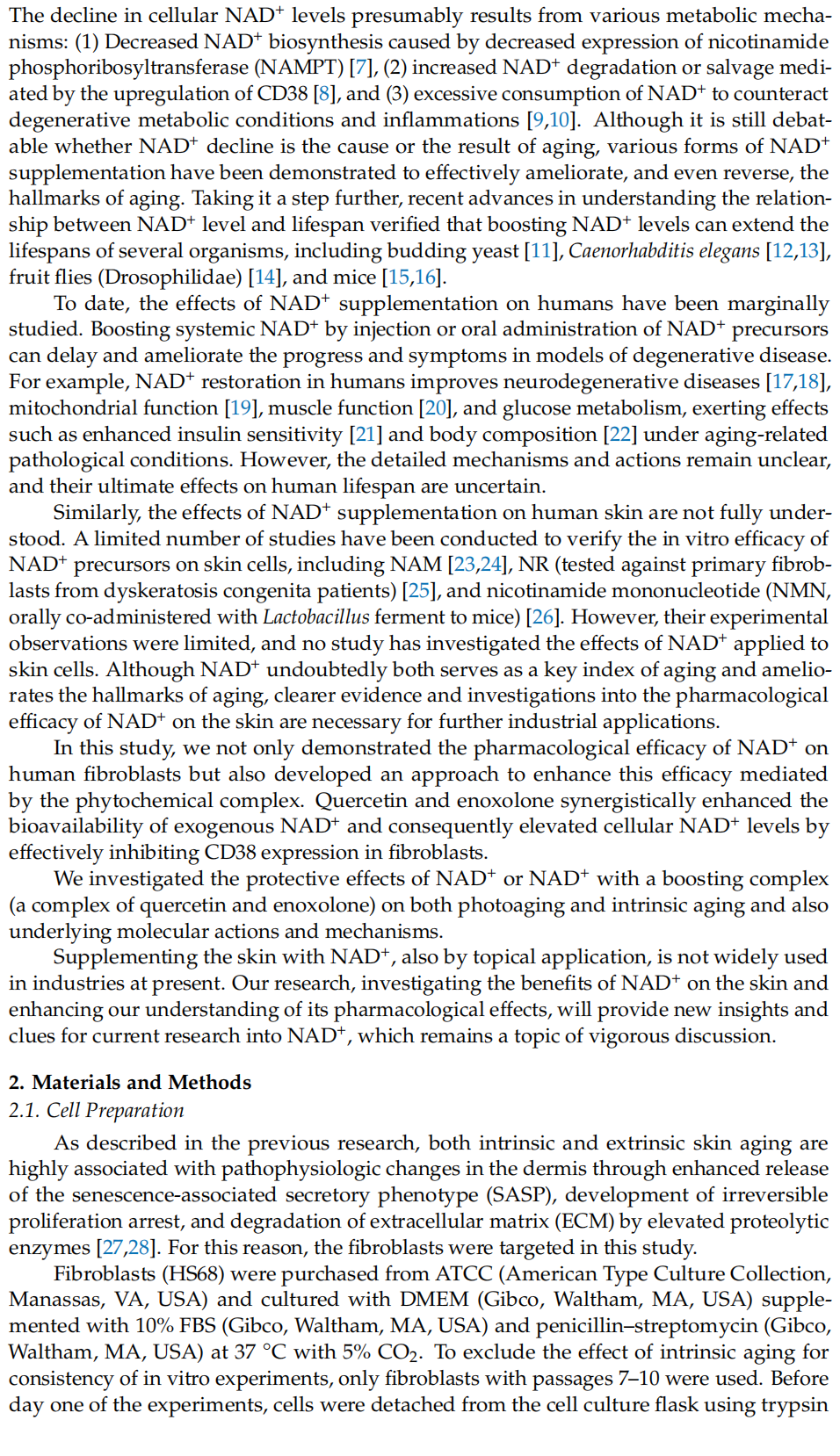





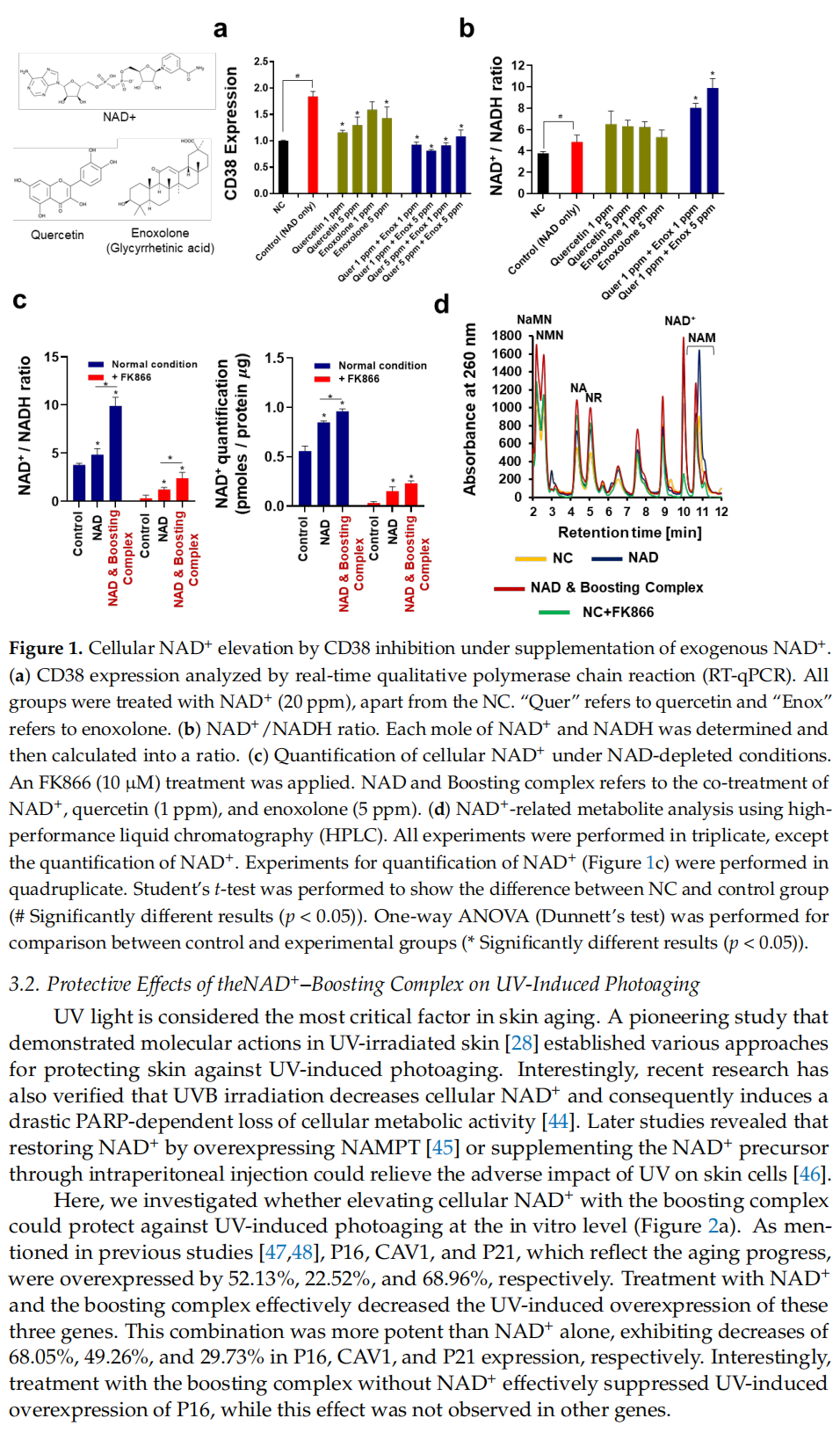
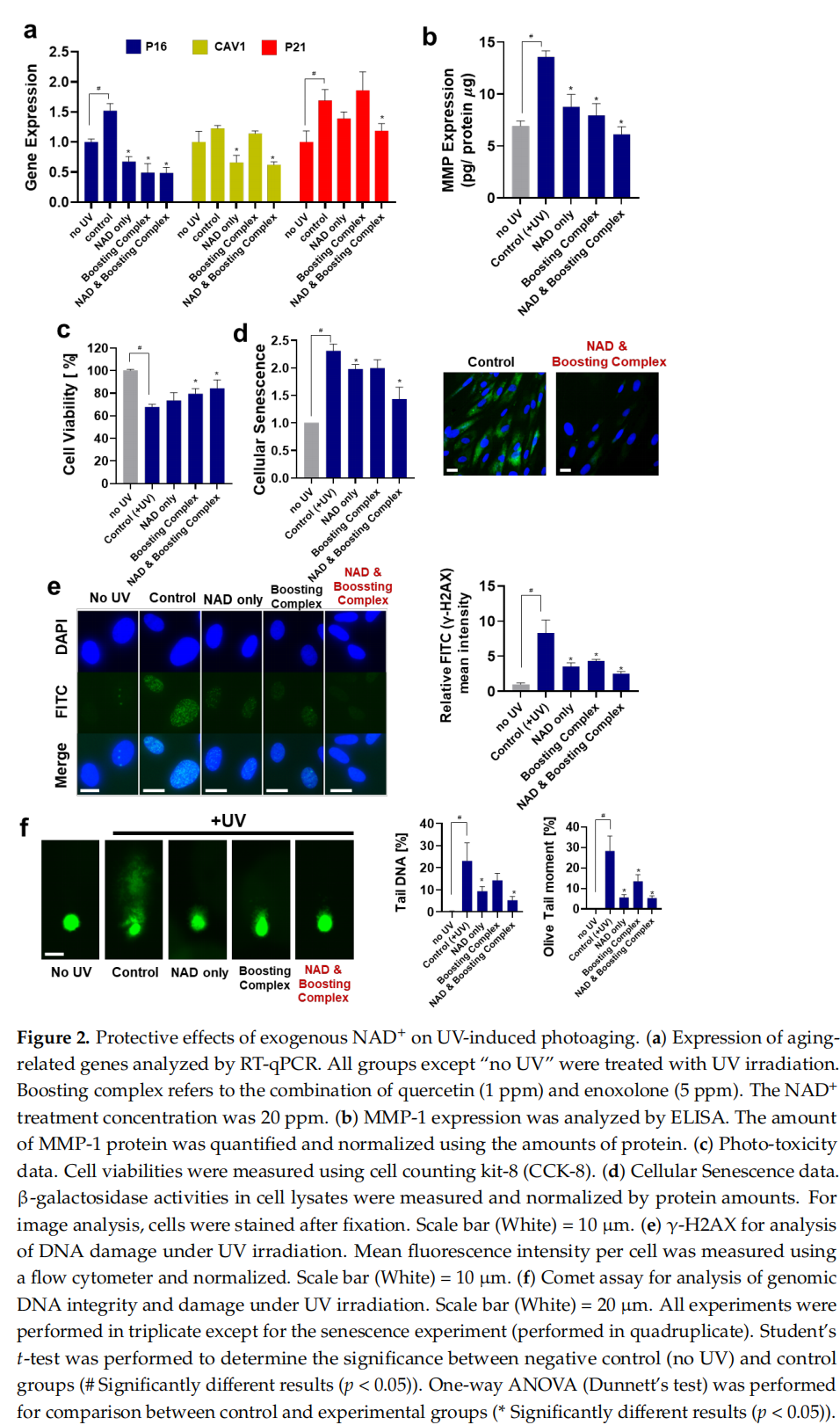



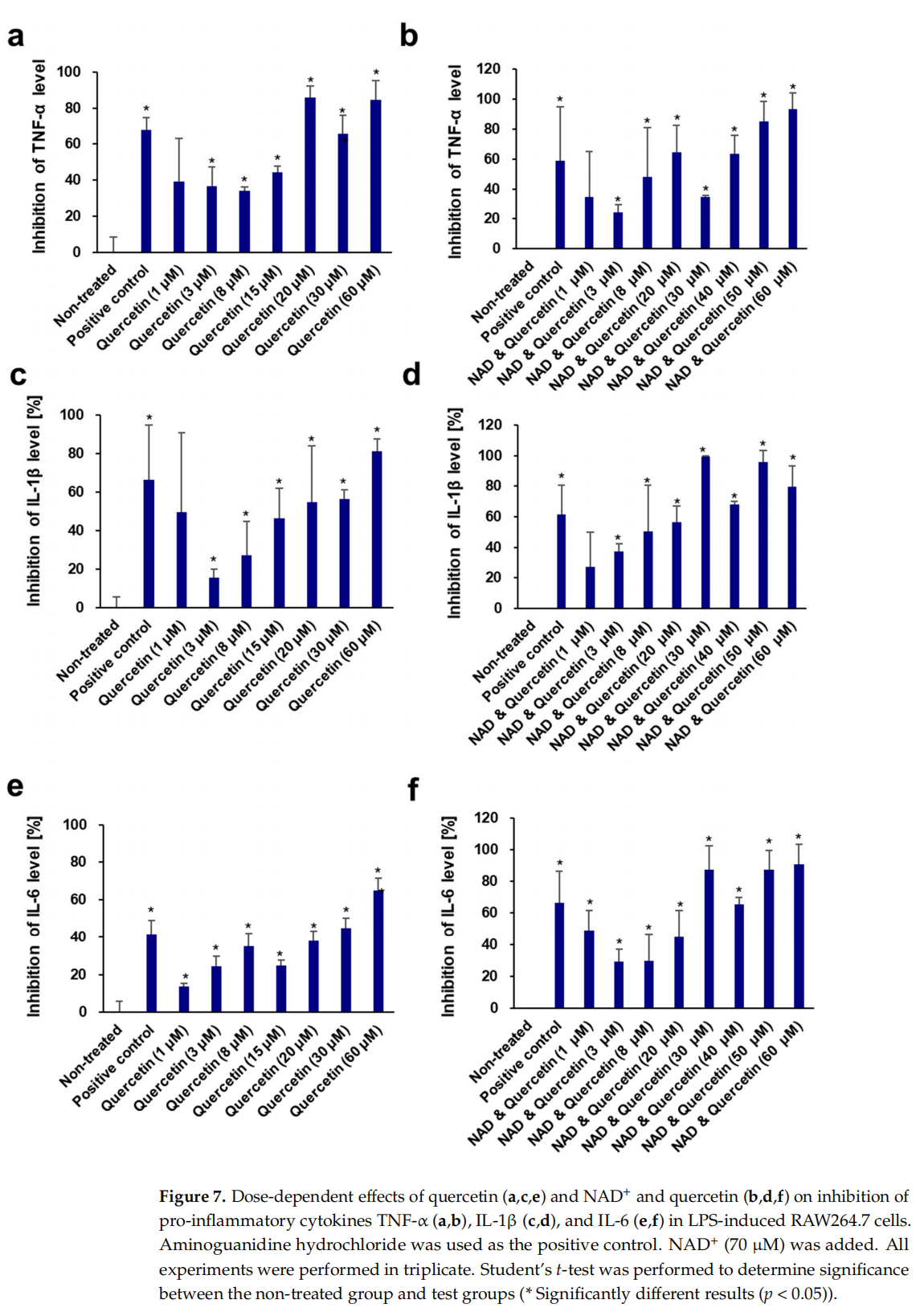
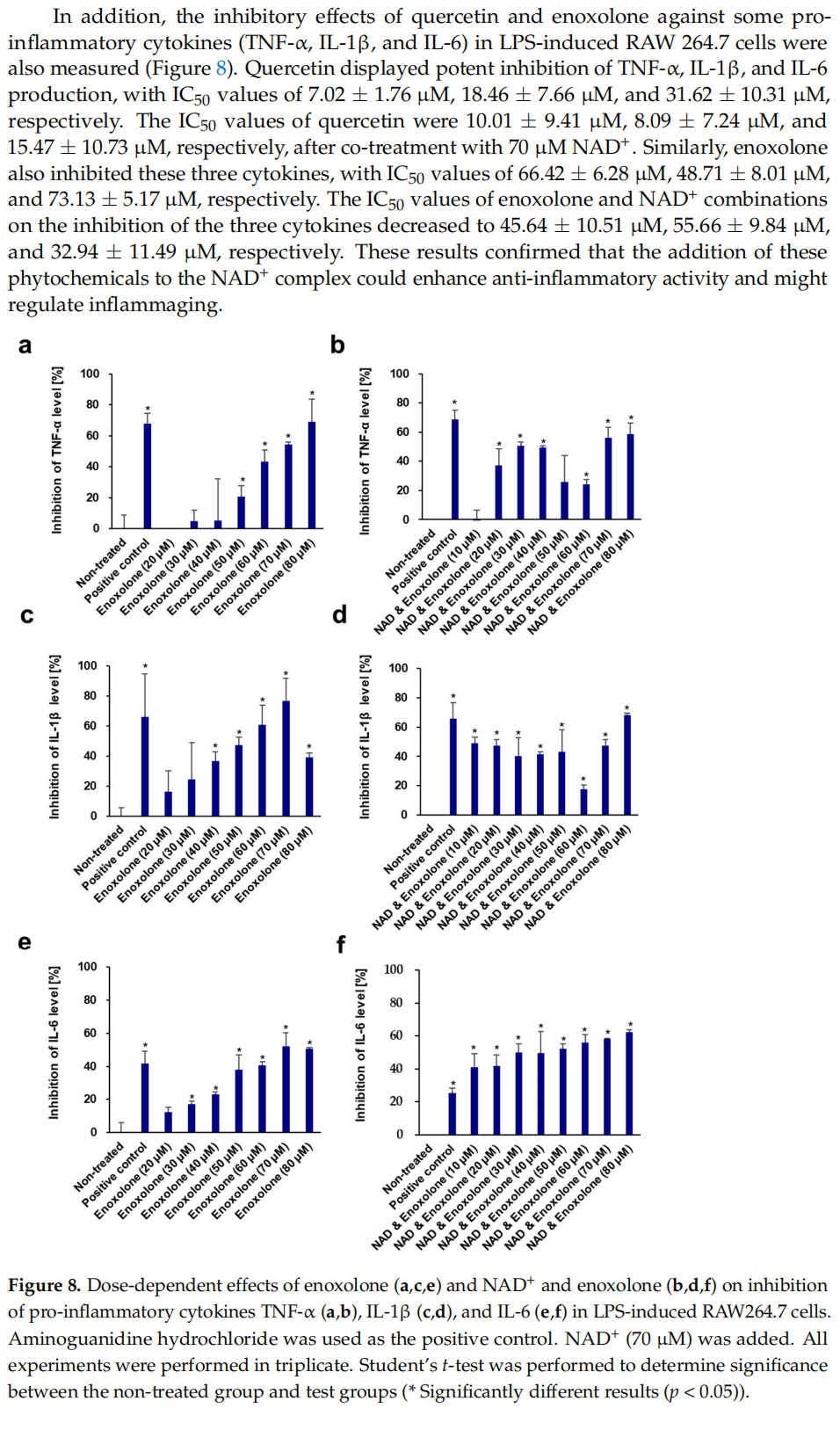





This article is excerpted from the Cells 2024, 13, 1799 by Wound World.

